eISSN: 2469-2794


Clinical Paper Volume 7 Issue 4
1Laboratory research, Federal University of Mato Grosso (UFMT)-Cuiaba, MT, Brazil
10Section of Biomedical Sciences, Mycology Laboratory, Adolfo Lutz Institute (IAL), Brazil
11Laboratory of Pathogenic Yeasts, School of Dentistry, University of Sao Paulo (USP), Brazil
2University Center of Varzea Grande (UNIVAG)-Varzea Grande, MT, Brazil
3Institute of Bioscience, Federal University of Mato Grosso (UFMT)-Cuiaba, MT, Brazil
4Institute of Biosciencies - State University of Sao Paulo “Julio de Mesquita Filho” (UNESP)-Rio Claro, SP, Sao Paulo, Brazil
5Paulista University (UNIP), Campinas-SP, Brazil
6Official Forensics and Technical Identification of the State of Mato Grosso (POLITEC)-Cuiaba, MT, Brazil
7State University of Campinas (UNICAMP), Campinas-SP, Brazil
8Department of Protection and Animal Welfare -Secretariat of Green Environment and Sustainable Development of Campinas, Prefecture of Campinas, Brazil
9Section of Mycology, Adolfo Lutz Institute (IAL), Sao Paulo-SP, Brazil
Correspondence: Diniz Pereira Leite Junior, Faculty of Medicine-Federal University of Mato Grosso-Cuiaba, MT, Brazil, Tel +55(65)98151- 9078
Received: July 21, 2019 | Published: July 29, 2019
Citation: Júnior DPL, Dantas ESO, Nascimento DC, et al. Action of fauna and flora on the cadaveric phenomena observed in the carcass of sus scrofa(Linnaeus-Suidae) in the wild area Brazilian savannah of the central region-Brazil. Forensic Res Criminol Int J. 2019;7(4):185-199. DOI: 10.15406/frcij.2019.07.00285
Decomposition is the process of cadaver degradation into its respective basic constituents by action of biological (microorganisms and arthropods) and abiotic (environmental conditions) agents. The objective was to know the richness, abundance and succession of entomological and fungal species with emphasis on the forensic importance in carcass of Sus scrofa in Brazilian Savannah of the central region Brazil. In this work, samples were collected and the action of biological agents was observed during putrefaction of experimental model. Overall, 5,009 insects specimens were collected, belonging to 3 orders, 15 families, 22 subfamilies, 39 genera and 47 species. Diptera was the most representative order, with 2,848 individuals (56.9%), followed by Hymenoptera with 1,628 (32.5%) and Coleoptera with 533 (10.6%). Diptera were present in all phases of cadaveric decomposition, of which, the butyric fermentation phase was the most relevant (26.6%). Hymenoptera were also present in the butyric fermentation phase (15.8%) and Coleoptera were present in the final phase of decomposition (7.8%). Regarding fungi, 223 specimens were isolated.
Four orders of filamentous fungi were identified: Eurotiales (44.4%), Mucorales (14.8%), Hypocreales (8.1%), emphasizing the presence of Aspergillus terreus. Among yeasts, the order Saccharomycetales (9.9%), represented by the genera Candida, Rodothorula and Pichia, and the order Tremellales (1.3%), represented by the Trichosporon genus, and were isolated. These microbiological entities were collected during all phases of cadaveric phenomena, highlighting the active decay period, when 26% of CFU’s were obtained. The skin was the anatomic site with the highest number of isolations (22.4%), followed by genital and perianal mucosa (17.5%), respectively. This study, which comprises the cadaveric biota, is extremely important as an elucidation tool. Forensic mycology is a rich field, where fungi can Interact and provide information, promoting the study of time of death in forensic cases.
Keywords: forensic sciences, thanatology, forensic entomology, forensic mycology, cadaveric fauna and flora, microbiology
Cadaveric phenomena are a set of transformations through which the human body goes through after death. The time passed since the cessation of bodily functions and the forensic examination, called postmortem interval, is not easy to be determined due to body cooling and dehydration as well as environmental and biological conditions.1 After death, a series of cadaveric transformations begin, and these decomposition processes can be classified into physical, chemical and biological mechanisms. According to authors2‒4 physical decomposition phenomena are dehydration, cooling and postmortem lividity. Chemical phenomena are autolysis, muscular rigidity, putrefaction, maceration, as well as conservative processes: mummification, saponification (adipocere) and calcification. The consecutive or mediate abiotic phenomena (due to the installation of cadaveric phenomena) are cadaveric dehydration or drying, lividity or patches of cutaneous hypostases (livor mortis), cadaveric cooling (algor mortis), cadaveric stiffness (rigor mortis) and cadaveric spasms. The transformative phenomena are, in turn, subdivided into destructive transformations (autolysis, putrefaction and maceration) and conservative transformations (mummification, saponification and calcification).4‒7 All these concepts merge into the science of life, seen through the perspective of death called Biothanatology.
In this work, destructive transformative phenomena will be studied, and their relationship with the action of biological agents. Putrefaction is the degradation of the tissue by the activity of a myriad of microorganisms, such as viruses, bacteria, fungi, protozoa, parasites and the toxins that some of these organisms produce.8 It presents distinct phases called chromatic or staining period, emphysematous or gaseous/deformative period, coliquative or melting period and the final phase called skeletonization period. Several authors9‒11 define that development of the body putrefaction period occurs due to the agreement between intrinsic factors (age, cause of death, physiology) and extrinsic (temperature, aeration, air humidity). These authors8‒11 also emphasize that although not having a rigorous chronology, cadaveric decomposition is done in five periods, phases or stages:
The duration of each phase in the decomposition process can undergo great variation. The climatic differences of each region associated with ambient temperature and air humidity, makes it almost impossible to establish precise intervals for the decomposition phases.
This study is important because it links fauna (entomology) and flora (mycology) in observations of the actions of cadaveric phenomena. The objective was to know the richness, abundance and succession of entomological and fungal species with emphasis on the forensic importance in carcass of Sus scrofa domesticus (Linnaeus, 1758). This is an observational study and identification of necrophagous agents, and their possible association with death estimates. This report is the initial starting point for the cadaveric biota, considered extremely important as a tool of elucidation, was studied. Insects were identified, with emphasis on the orders Diptera, Coleoptera and Hymenoptera (Formidae family). In contrast, forensic mycology is associated with the biotic group and has become a field rich in information. In this way, fungi can interact and provide information, helping the elucidation of the time of death involving forensic cases.
Mato Grosso, central region of Brazil, is the third largest state in the country and covers three biomes: The Amazon (rain forest), Cerrado (Brazilian savannah) and Pantanal (tropical wetland). The study was conducted in the locality of Jamacá in the city of Chapada dos Guimarães/Mato Grosso-Brazil, in a particular area located by the coordinates: GPS:L06-01-88-4/N82-77-22-8 (Figure 1).
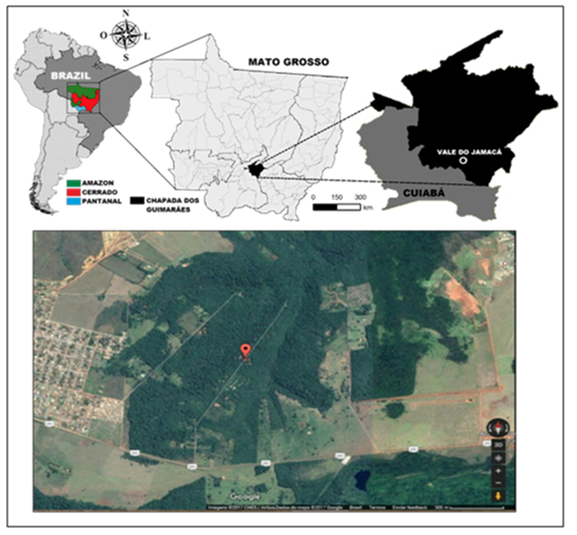
Figure 1 Identification of the biomes of Midwest Brazil and location of the experiment, Vale do Jamaca, Chapada dos Guimarães, Mato Grosso, Brazil (2018).
The study site consists of preserved vegetation, with the presence of different phytophysionomy: grass, herbs, shrubs and trees, composing a rich flora of the Cerrado/Brazilian Savannah (Riparian forest, gallery forest, dry forest, Cerrado (dense, typical and rupestrian), dirty field, clean field, Vereda and Palm Grove). With a lush biodiversity, the Cerrado is the main biome of the Midwest, and is predominant in the Chapada dos Guimarães.12 The experimental model used was the carcass of S. scrofa domesticus, with body weight of about 15kg, preserved inside a metal cage (60 X 90 X 45cm high). The carcass was deposited on a sand substrate that served as a pupation site for larvae in the hatching process. In this location, the chromatic, gaseous, colliquative and skeletonization periods were observed, defined in this study in five periods: fresh, gaseous; dark putrefaction; advanced putrefation (fermentation) and skeletonization11. The chosen animal was used because it is considered the best model for entomological analysis compared to humans due to the similarity in decomposition9 and internal characteristics of its organs, diet, skin, thoracic cavity and intestinal microbiota.13,14 The animal was acquired by a local breeder in the vicinity of the study area, which commercialized animals intended for human consumption. The animal model used in the study was put down at 05:00 a.m. of the first day of the Experiment (Day 0), transported intact and preserved to the place where the decomposition process would occur as well as the collection of the entomological and fungal specimens (Figure 1). Shortly after the carcass was placed in the experimental area, after 3hours of deposition, the collections initiated and were carried out every day until the carcass was found in complete skeletonization. Licenses from the Research Ethics Committee were not required at the time of the experiment and the ethics committees were not available for any corresponding evaluation. In this case, since it involves the consumption of animal meat, it is considered a legal practice to slaughter animals for commercialization in local fairs in the region of the research.
According to the climatic system of Köppen-Geiger, Mato Grosso is characterized as Cwa: subtropical, dry winter and rainy summer.15 The climate is characterized by the semi-arid region (hot and humid), with average annual precipitation of 1,500mm and average annual temperature between 25°C and 40°C. Despite this inequality, the region is well supplied with rain and seasonality and is considered typically tropical, with maximum values in summer and minimums in winter, with two distinct seasons: a dry season (fall and winter) that extends from April to September, with about 20% of the total annual rainfall; and a rainy season (Spring and Summer), which extends from October to March, with more than 80% of the total annual rainfall. When cooling occurs, which is the inversion of the polar mass on the continent; there may be a decrease in temperature.16,17 At the site of the experiment, during the 17days of biological material collection of both entomofauna and mycological samples, two digital thermo-hygrometers were used, in pairs, with reading capacity from −10 to +60oC (Model 7429.02.0.00 Brand Incoterm). The devices were used for the collection of environmental temperature (°C), as well as relative air humidity (%) data (Figure 2).
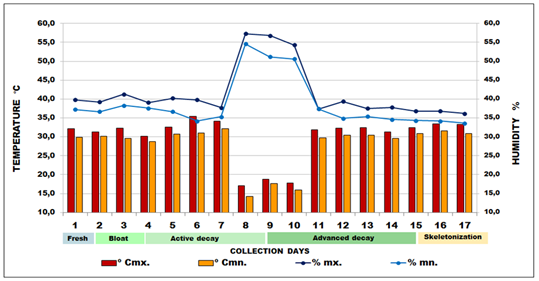
Figure 2 Daily mean for the relative humidity (RH%) and Ambient Temperature (ºC) for the collection site in Vale do Jamacá, Chapada dos Guimarães, Mato Grosso-Brazil (2018).
0Cmx. = maximum temperature; 0Cmn. = minimum temperature; %mx. = maximum humidity; %mn. = minimum humidity.
Cadaveric entomofauna
The total duration of the death process until skeletonization was 408hours, or 17days (21/July to 06/August/2018). Entomological samples were collected from a specimen of S. scrofa in the periods of cadaveric phenomena. On the first day, samples were collected after 3hours of carcass deposition. During the remaining 16 days, the samples were collected exactly from 7:30 to 9:00 in the morning in order to avoid the warmer times, and the decomposition phases were observed and recorded according to the literature until the complete decomposition of the experimental model11. For the collection of Diptera, the larvae were collected with the aid of entomological tweezers, choosing only well-developed larvae on the substrate. These specimens were transported alive in plastic pots with damp cotton, and the lid was kept ajar for oxygenation. Some winged adult specimens, which flew over the carcass, were collected with the help of an entomological net and transported in vials containing 70% alcohol. Regarding Coleoptera and Hymenoptera (formidae), mostly adult specimens were collected during direct observation of insect activity on the carcass and monitoring of pitfall traps. For the collection of crawling insects, twelve pitfall traps were used, consisting of a 2-liter plastic bottle with 15cm diameter by 30cm of height, containing 1,000mL of water, 2mL of 4% formaldehyde, and 20mL of liquid detergent18,19 to preserve arthropods.
The traps were arranged around the cage with the carcass, where they were divided into four quadrants according to the orientation of the cardinal points (three pitfalls per quadrant), and buried 30cm deep, in circular shape,20 at an approximate radius of 1.5m of the animal and with 1.0 m distance between traps. The traps were emptied every three days to accompany the succession of insects. Some specimens of Hymenoptera were collected with the help of tweezers and brushes and transported to the laboratory in microtubes containing 70% ethanol11. For identification, the specimens collected (diptera, coleoptera and hymenoptera) were transported to the Entomological Laboratory located in Federal University of Mato Grosso. The larvae collected (diptera) completed their life cycles, necessary for identification, were euthanized with the aid of ethyl acetate, submerged in Dietrich Fixative Solution (distilled water, 95% alcohol, formaldehyde, acetic acid and glycerin), used as a fixer to maintain the integrity of colors and structures of the collected specimens.19 After storage in the solution, all entomological fauna was preserved, screened, and identified by the morphological traits for species description. For this purpose, we used manual magnifying glasses, stereoscopic microscope (model SZ51 8x-40x, Olympus, Brazil), dichotomous keys and species records recommended by several authors.21‒39
Cadaveric mycobiota
Parallel to the entomological collection, the samples for fungal identification were collected during the 17days of the cadaveric decomposition process. For this study, a total of 84 samples containing mycological material were collected. The samples were collected with the aid of sterile swabs, through friction on oral, nasal, auditory, perianal and genital mucosa40 and animal skin (dorsum and abdomen). For each anatomical site, a swab was used in order to demonstrate fungal growth and isolation. These anatomical sites are commonly analyzed in laboratory mycological diagnosis and were chosen because they were more likely to demonstrate fungal growth.40 In the first 11 days of collection, six swabs were used in the sites: oral, nasal, auditory, genital, perianal and skin. On the 12th and 13th day, five swabs were used: oral, nasal, genital, perianal and skin, because the auditory canal no longer existed. Finally, in the skeletonization phase, the mucous membranes ceased to exist due to entomological necrophagy, so the collections occurred only on the skin and bones of the carcass. For primary isolation and fungal identification, the swabs were gently rubbed over 70mm diameter petri dishes containing Sabouraud Dextrose Agar (SDA) (DIFCOTM) and chloramphenicol (100mg/mL), seeded and incubated at room temperature for 7 to 10days for fungal growth. Then, the filamentous fungi and yeasts were isolated in other containers containing the same medium of primary isolation. For filamentous fungi, after secondary growth, the morphological study (macroscopic and microscopic) was performed in SDA medium with chloramphenicol (100mg/mL), Malt Extract Agar (MEA), Potato Dextrose Agar (PDA) and Czapek-Dox Broth Agar (CZA) (DIFCOTM) and proceeded to the verse and reverse observation of the colonies, especially its pigmentation. For this step, the isolated fungi were seeded in a petri dish (70mm diameter) for better observation.
For each colony, a microculture was performed on PDA and incubation at room temperature for 10 days. After this period, the Riddell Technique41 was used, where the cover slips were removed and placed on another sterile slide with a drop of lactophenol cotton blue (LPCB) dye, and the morphology observed under light microscope with 40x objective. The identification was based on the reference literature.41‒49 For the study of micromorphological features of yeasts, after isolation, the colonies were tested for their purity by plating in BBLTM CHROMagarTM Candida chromogenic medium to presumptively identify and differentiate fungi according to the morphology and color of the colonies. After that, the microculture technique (Riddell Technique),41 was used in Corn Meal Agar (CMA) with Tween 80 medium. This technique allows the identification of genera, or even species of yeasts through the analysis of the presence and disposition of structures such as blastoconidia, arthroconidia, hyphae and pseudohyphae.46 Urease test was also used as a presumptive form of biochemical identification in Christensen's medium for the detection of urease enzyme produced by fungi.
In Brazil, a country of great biological diversity and extensive territorial dimension, the assessment of the succession patterns of entomological fauna and flora is challenging, since it presents great climatic differences and several Biomes.50 The Brazilian cadaveric entomological fauna presents great diversity. We can highlight the great frequency and abundance of insects, as well as knowledge of information related to them, such as: taxonomic identification, biological cycle, geographic distribution of species and their ecological interactions. This set allows the application of entomology to aid in judicial investigations and as a criminalistics tool, for example, in Postmortem interval estimation.11,50,51 Among the 5.009 immature and adult insects collected, 3 large orders stood out: Diptera (2,848; 56.9%), Hymenoptera (1,628; 32.5%) and Coleoptera (533; 10.6%) distributed among 15 families, 27 subfamilies, 40 genera and 46 species and 223 fungal specimens, distributed in three phylla, 12 orders, 21 genera and 35 species, were identified (Figure 3).
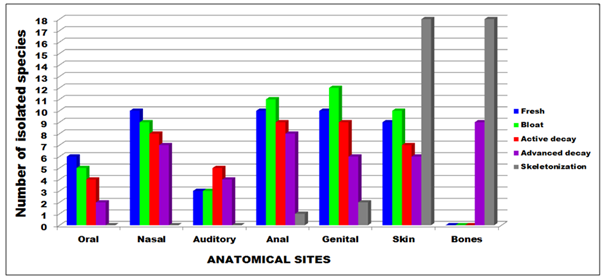
Figure 3 Flutuation of necrophagous insects (diptera, coleoptera and hymenoptera) and fungi collected during the decomposition stages in Vale do Jamacá, Chapada dos Guimarães, Mato Grosso, Brazil (2018).
There are two orders of insects with great forensic importance: Diptera and Coleoptera.13‒54 Overall, the most abundant order was Diptera, with (56.9%), and the families with the largest number of diptera specimens identified were: Calliphoridae (67.9%), Muscidae (21.0%), Sarcophagidae (5.3%), Fannidae (5.0%), Tabanidae (0.5%) and Drosophilidae (0.2%). Among coleopterans, specimens of the following families were identified: Cleridae (30.4%), Dermestidae (26.5%), Nitidulidae (14.6%), Hysteridae (8.6%), Staphylinidae (5.8%), Silphidae (4.3%), Cincidelidae (3.9%) and Scarabaeidae (3.0%). The identified Hymenoptera were represented by the Formicidae family, including the subfamilies: Myrmicinae (36.5%), Formicinae (25.5%), Ectatomminae (18.9%), Ponerinae (15.5%) and Paraponerinae (3.6%) (Table 1).
DIPTERA |
(N) total: 2,848 flies |
PHASES |
|
||||||
Families |
Subfamilies |
Species |
I |
II |
III |
IV |
V |
N |
% |
Calliphoridae |
Chrysomyinae |
Chloroprocta ideoidea |
10 |
15 |
27 |
25 |
5 |
82 |
1,6 |
Chrysomya albiceps |
52 |
79 |
92 |
51 |
28 |
302 |
6,0 |
||
Chrysomya megacephala |
28 |
112 |
61 |
33 |
21 |
255 |
5,1 |
||
Chrysomyia putoria |
28 |
41 |
42 |
17 |
12 |
140 |
2,8 |
||
Cochliomyia hominivorax |
3 |
8 |
5 |
2 |
0 |
18 |
0,4 |
||
Cochliomyia macellaria |
28 |
57 |
27 |
31 |
10 |
153 |
3,1 |
||
Hemilucia segmentaria |
12 |
12 |
10 |
7 |
2 |
43 |
0,9 |
||
Calliphorinae |
Lucilia eximia |
32 |
78 |
76 |
21 |
19 |
226 |
4,5 |
|
Lucilia sericata |
39 |
86 |
101 |
45 |
13 |
284 |
5,7 |
||
Lucilia ilustris |
26 |
35 |
30 |
22 |
12 |
125 |
2,5 |
||
Lucilia porphyrina |
21 |
19 |
26 |
21 |
10 |
97 |
1,9 |
||
Lucilia cuprina |
22 |
42 |
32 |
23 |
11 |
130 |
2,6 |
||
Caliphora |
0 |
0 |
0 |
0 |
0 |
0 |
0,0 |
||
Toxotarsinae |
Sarconesia chlorogaster |
12 |
19 |
15 |
22 |
12 |
80 |
1,6 |
|
Muscidae |
Azellinae |
Ophyra albuquerquei |
36 |
52 |
12 |
32 |
23 |
155 |
3,1 |
Ophyra aenescens |
20 |
23 |
32 |
19 |
7 |
101 |
2,0 |
||
Ophyra solitária |
10 |
12 |
12 |
8 |
3 |
45 |
0,9 |
||
Muscinae |
Muscina stabulans |
5 |
6 |
3 |
2 |
2 |
18 |
0,4 |
|
|
Musca domestica |
39 |
117 |
63 |
42 |
17 |
278 |
5,6 |
|
Sarcophagidae |
Sarcophaginae |
Pechia intermutans |
10 |
8 |
10 |
10 |
6 |
44 |
0,9 |
Sarcophaga argyrostoma |
5 |
4 |
6 |
3 |
2 |
20 |
0,4 |
||
Sarcodexia lambens |
10 |
2 |
2 |
2 |
0 |
16 |
0,3 |
||
Oxysarcodexia thornax |
8 |
12 |
6 |
4 |
2 |
32 |
0,6 |
||
Oxysarcodexia amorosa |
9 |
21 |
5 |
3 |
2 |
40 |
0,8 |
||
Fanniidae |
|
Fannia pusio |
13 |
22 |
20 |
15 |
10 |
80 |
1,6 |
Fannia cannicularis |
13 |
15 |
25 |
8 |
2 |
63 |
1,3 |
||
Tabanidae |
Tabaninae |
Tabanus sp. (1) |
0 |
3 |
1 |
4 |
2 |
10 |
0,2 |
Tabanus sp. (2) |
0 |
1 |
2 |
1 |
0 |
4 |
0,1 |
||
Drosophilidae |
Drosophilinae |
Zaprionus indianus |
0 |
2 |
1 |
1 |
0 |
4 |
0,1 |
Drosophila spp. |
0 |
1 |
1 |
1 |
0 |
3 |
0,1 |
||
TOTAL |
491 |
904 |
745 |
475 |
233 |
2848 |
56,9 |
||
Coleoptera |
(N) total: 533 beetles |
Phases |
|||||||
Families |
Subfamilies |
Species |
I |
II |
III |
IV |
V |
N |
% |
Dermestidae |
Dermestinae |
Dermestes maculatus |
6 |
5 |
15 |
17 |
42 |
85 |
1,7 |
Dermestes spp. |
4 |
6 |
10 |
9 |
27 |
56 |
1,1 |
||
Histeridae |
Histerinae |
Hister spp. |
2 |
4 |
6 |
13 |
21 |
46 |
0,9 |
Cleridae |
Clerinae |
Necrobia rufipes |
10 |
9 |
12 |
32 |
24 |
87 |
1,7 |
Necrobia ruficollis |
8 |
5 |
5 |
10 |
20 |
48 |
1,0 |
||
Necrobia spp. |
0 |
2 |
5 |
12 |
8 |
27 |
0,5 |
||
Nitidulidae |
Nitidulinae |
Stelidota geminata |
0 |
2 |
4 |
8 |
20 |
34 |
0,7 |
|
Nitidula carnaria |
1 |
5 |
6 |
12 |
20 |
44 |
0,9 |
|
Scarabaeidae |
Scarabaeinae |
Aphodius spp. |
0 |
2 |
3 |
4 |
7 |
16 |
0,3 |
Silphidae |
Silphinae |
Oxelytrum cayennense |
0 |
2 |
4 |
2 |
3 |
11 |
0,2 |
Oxelytrum discicolle |
0 |
4 |
5 |
3 |
0 |
12 |
0,2 |
||
Cincedelidae |
Cincidelinae |
Tetracha brasiliensis |
1 |
4 |
7 |
4 |
5 |
21 |
0,4 |
Staphylinidae |
Aleocharinae |
Aleochara spp. |
1 |
0 |
3 |
3 |
8 |
15 |
0,3 |
Staphylininae |
Belonuchus rufipennis |
1 |
2 |
2 |
4 |
10 |
19 |
0,4 |
|
Philonthus spp. |
0 |
1 |
0 |
3 |
8 |
12 |
0,2 |
||
|
Total |
34 |
53 |
87 |
136 |
223 |
533 |
10,6 |
|
Hymenoptera |
(N) total: 1,628 ants |
|
|||||||
Family: Formicidae |
Phases |
||||||||
Subfamilies |
Tribes |
Species |
I |
II |
III |
IV |
V |
N |
% |
Myrmicinae |
Solenopsidini |
Solenopsis invicta |
29 |
50 |
35 |
36 |
20 |
170 |
3,4 |
Solenopsis saevissima (C.) |
13 |
15 |
31 |
23 |
23 |
105 |
2,1 |
||
Pheidolini |
Pheidole spp. |
15 |
10 |
18 |
28 |
16 |
87 |
1,7 |
|
Crematogastrini |
Crematogaster spp. |
18 |
12 |
18 |
21 |
15 |
84 |
1,7 |
|
Attini |
Atta spp. |
0 |
8 |
15 |
13 |
12 |
48 |
1,0 |
|
Acromyrmex subterraneus |
16 |
18 |
22 |
31 |
29 |
116 |
2,3 |
||
Acromyrmex spp. |
12 |
9 |
16 |
18 |
21 |
76 |
1,5 |
||
Formicinae |
Camponotini |
Camponotus rufipes |
21 |
35 |
52 |
39 |
21 |
168 |
3,4 |
Camponotus melanoticus |
22 |
35 |
48 |
31 |
20 |
156 |
3,1 |
||
Paraponerinae |
Paraponerini |
Paraponera clavata |
8 |
11 |
9 |
24 |
7 |
59 |
1,2 |
Ponerinae |
Ponerini |
Dinoponera gigantea |
2 |
16 |
20 |
17 |
15 |
70 |
1,4 |
Dinoponera grandis |
0 |
12 |
16 |
32 |
11 |
71 |
1,4 |
||
Odontomachini |
Odontomachus spp. |
16 |
18 |
25 |
34 |
18 |
111 |
2,2 |
|
Ectatominae |
Ectatommini |
Ectatomma edentatum |
7 |
17 |
34 |
47 |
18 |
123 |
2,5 |
Ectatomma opaciventre |
6 |
18 |
32 |
33 |
21 |
110 |
2,2 |
||
Ectatomma spp. |
0 |
12 |
17 |
23 |
22 |
74 |
1,5 |
||
(N) overall total: 5,009 insects |
|
Total |
185 |
296 |
408 |
450 |
289 |
1628 |
32,5 |
Table 1 Distribution of insect species (diptera, coleoptera and himenoptera) collected on Sus scrofa (Linnaeus) carcass in vale do Jamacá, Chapada dos Guimarães, Mato Grosso, Brazil (2018).
Also, other orders were observed: Lepidoptera, Hemiptera, Orthoptera, Isoptera and Dermaptera. These insects were observed but were not captured nor summarized because they were considered visitors and due to the small number of specimens compared to the other taxa. Within the order Diptera, the most important families are: Calliphoridae, Sarcophagidae, Muscidae, Fanniidae e Stratiomyidae.9,11,25,50‒55 Several authors25,50 established that in South America, in addition to the families Calliphoridae, Sarcophagidae and Muscidae, other families of Diptera have forensic interest: Drosophilidae, Phoridae, Anthomyiidae, Sphaeroceridae, Sepsidae, Ulidiidae, Piophilidae, mostly because they present necrophagous habits and are often found in carcasses and cadavers. A total of 2,848 muscomorph individuals were collected and represented by 2,108 adults and 740 larvae, which developed in the laboratory, constituting six families:
Calliphoridae family was the most observed, represented by three subfamilies: Chrysomyinae (Chloroprocta ideoidea,27 Chrysomya albiceps (Wiedemann, 1819), C. megacephala (Fabricius, 1794), C. putoria (Wiedemann,1830), Cochliomyia hominivorax (Coquerel,1858), C. macellaria (Fabricius, 1775), Hemilucilia segmentaria (Fabricius, 1805), Calliphorinae (Lucilia eximia (Wiedemann, 1819); L. sericata (Meigen, 1826); L. ilustris (Meigen, 1826); L. porphyrina (Walker, 1856); L. cuprina (Wiedemann, 1830) and Caliphora sp.) and Toxotarsinae (Sarconesia chlorogaster (Wiedemann, 1830)).
The Muscidae family was the second most abundant, with representatives of the following subfamilies:
Members of the families Fanniidae (Fannia cannicularis (Linnaeus, 1761) and Fannia pusio (Wiedemann, 1830)), Tabanidae, represented by the subfamily Tabaninae (genus Tabanus) and Drosophilidae, represented by subfamily Drosophilinae (Zapionus indianus (Gupta, 1970) and Drosophila spp.) were also observed. On the other hand, current studies point out that there is no exact definition of the moment when each species will appear during cadaveric decomposition. In the last decades, the use of flies as indicators of death has helped in directing investigations in cases of murders.51 According to Vanrell56 the most frequently observed insects in necrophagic fauna are Diptera, which can guide or assist in determining the approximate date of death in cases of discovery of human bodies, due to the chronology, succession and duration of their life cycle. In a study conducted in the city of Cuiabá/MT57 observed specimens of Diptera on a swine carcass, which belonged to the families Calliphoridae, Syrphidae and Muscidae. The most abundant in this study was the family Calliphoridae. Recently, in the same city, Dantas and his collaborators16 identified 15 species of Diptera, distributed by the families Muscidae, Calliphoridae and Sarcophagidae, again highlighting the family Calliphoridae. The abundance of Diptera reached its peak between the 3rd and 7th days, corresponding to the phases of bloating and dark putrefaction.
Representatives of Calliphoridae, Muscidae and Sarcophagidae families were the most prevalent specimens of flies colonizing the carcass. The 2,848 muscomorphs were captured on the carcass from the first to the last day of the study. It was possible to detect a decrease in the number of individuals during the skeletonization phase due to the low supply of decaying tissue. During the fresh phase, Calliphoridae dominated, especially Lucilia cuprina, L. eximia, Chrysomyia albiceps and L. porphyrina. During bloat phase, it was possible to observe a predominance of Musca domestica, followed by Chrysomyia megacephala. During putrefaction, C. albiceps and L. sericata prevailed. On the fermentative phase, C. albiceps was most abundant, while on skeletonization phase, L. sericata. Muscidae are species off lies found mainly in high plasticity, domestic environments, associated with humans.54 Musca domestica, was the second most abundant species (278; 5.6%) and was identified on the experimental organism every day. This highly present species peaked between the 2nd and 4th days, being detected after approximately 18 hours after the death of the pig. During this period, other members of the Muscidae family were identified: Ophyra albuquerquei (155; 3.1%) and O. aenescens (101; 2.0%), which were present on the carcass until the last day of decomposition (Table 1).
There are several genera of Sarcophagidae found in carcasses in Brazil, of which: Sarcophaga (Liopygia), Sarcophaga (Bercaea), Peckia (Euboettcheria), Peckia (Pattonella), Peckia (Peckia), Peckia (Squamatodes), Dexosarcophaga, Sarcodexia, Oxysarcodexia, Helicobia, Ravinia and Trichaera can be listed.11 These muscomorphs are larviparous, meaning they lay 1st instar larvae and, according to Gennard,53 they prefer more advanced stages of decomposition. These necrophagous specimens were more present from the 7th and 8th day of decomposition. Members of the Sarcosaprophage family, even though collected throughout all 17 days of carcass decomposition, did not reach abundance as high as that observed for Calliphoridae. Identified species were: Peckia (Pattonella) intermutans (44, 0.9%), which is larviparous and pioneer in decomposition processes28,58 followed by Oxysarcodexia amorosa (40, 0.8%) and O. thornax (32, 0.6%) (Table 1). Regarding the family Fanniidae, its members are frequently associated with decomposing animal matter.59 In this study, Fannia pusio and Fannia cannicularis were identified. The most important species occurring in Brazil are Fannia pusio (Wiedemann, 1830) and Fannia cannicularis (Linnaeus, 1761),11,53 which in the present study were also isolated: Fannia pusio (80; 1.6%) and Fannia cannicularis (63; 1.3%) (Table 1).
In this study, the whole process of cadaveric decomposition happened in 17 days, until the skeletonization period. The observations made for the description of the decomposition processes are in conformity with the five stages (phases) in which all corpses pass, presenting a similar pattern of decomposition, and which were advocated.9,10,11 During the experiment, the highest temperature recorded at the site was 35.6°C on the sixth day, and the lowest was 17.1°C on the eighth day, due to occurrence of a sudden drop in temperature in the region. The variation in ambient temperature over the sampling period was relatively moderate in terms of what is expected for the region. Regarding local humidity, it peaked at 57.3% and fell as low as 36.2% in the last day (Figure 2).
The fresh phase (initial decomposition) occurred in the first 31hours (1st to 2nd day). Then, the bloat phase lasted 42hours (2nd to 3rd day), followed by the dark putrefaction phase for 120 hours (4th to 8th day). In the night of the eighth day, there was a decrease in temperature (17.1ºC) and moderate to strong rainfall (57.3%). On the next day, butyric fermentation started, which lasted 144 hours (9th to 14th day); the temperature drop was also perceived in the 9th and 10th days, which inhibited adult necrophilic insect activity. Only the larvae remained active inside the holes and wounds of the carcass. Few insects could be seen flying over the carcass during collection on those three days. Oliveira-Costa,11 state that rain does not affect larvae activity in the cavities of the decomposing body, which could be observed also in this research. On the 11th day, the temperature rose, remaining constant until the end of the experiment. From the 14th to the 17th day, the dry phase or skeletonization began, totaling 72hours, after which, collection was halted. After the last collection of the 17th day, it was possible to observe the total desiccation of the experimental model (Figure 2).
The decomposition rate can be different even in the same area or district, due to regional, environmental, topographical and geographical factors.13 Dias57 reported the skeletonization of a Sus scrofa carcass after 16days, in the city of Cuiabá, Mato Grosso. It was possible to observe that, for the studied region, active decay started on the fourth day after death, and advanced decay, after the ninth day. The dry remains phase was identified on the 15th day, when the final collection events took place up to the 17th day. Specimen collection was avoided around 10:00 a.m. due to the extreme heat and low winged insect activity, even in the presence of shade from the vegetation, where thermal sensation was 40ºC. Several authors have reported that biotic phenomena are affected by the environment (rain, heat, cold, humidity...)1,7,13 The temperature drop observed in the present study could have slowed down necrophagy and decomposition, which resumed as the temperature rose again on the next days. The rain may also have slowed down tissue dehydration, hampering the decomposition process. Temperature influences larvae activity on the carcass13,52,53 including that larval activity by itself may increase temperature through production of metabolic heat. The mentioned authors also report that high temperatures increase the number and type of insects associated with the cadaver and their activity accelerates decomposition.
In that context, since the research was conducted in months of intense heat in the region, the decomposition process may have been stimulated, also contributing to the overwhelming quantity of necrophagous insects observed. During this study, the presence of Tabanids, which are flies of sanitary, medical and veterinary importance, was noted. The region where this research was conducted exhibits very strong presence of these mechanical pathogen agents, despite the low presence reported in this study. We can point out that this group of flies possesses forensic importance. The order Coleoptera (beetles) is the second insect group of highest forensic interest in Brazil, due to its large number of described species.21,26,55 This order is composed by several families, such as the forensically important Silphidae and Dermestidae. Other families are also attracted to the latter stages of decomposition: Hysteridae, Staphylinidae, Cleridae and Nitidulidae families.60 During this research, 533 beetles were collected, distributed in eight distinct families, nine subfamilies, 11 genera and 9 species (five different groups were identified only at the gender level). Of these, 221 specimens were collected during the drier period. Specimens belonged to the following species according to their order of abundance: Cleridae (163; 30.6%), Dermestidae (142; 26.6%), Nitidulidae (80; 15%), Hysteridae (46; 8.6%), Staphylinidae (27; 5.1%), Silphidae (23; 4.3%), Cincidelidae (21, 3.9%) and finally, Scarabaeidae (16, 3.0%) (Table 1).
From the eleventh day, a larger number of Coleoptera was found in the experimental model. In addition, the decomposition process was finished in seventeen days due to the influence of the hot season in the region (Table 1). The activity of coleopterans began on the third day and increased on the sixth day. The number of beetles on days 2 and 3 was low (<20), rising with the course of the experiment up to days 7 and 8. This coincided with the final phase of the dark putrefaction stage and the beginning of the fermentative phase. Coleopterans were identified every day, especially after the fourth day but not around days 8 through 10, due to rainfall and temperature drop. The pattern of succession of beetles progressed according to their ecological roles. Beetles, categorized both as carrion feeders and predators of Diptera, including Cleridae, Hysteridae, Staphylinidae and Silphidae, arrived after larvae were present in the body. Before that, in the fresh phase, it was only recorded the presence of the families: Dermestidae, Hysteridae, Cicindelidae and Staphylinidae, all in low number (≤1). The arrival of these taxa occurred in greater intensity in the fourth or seventh day, when the carcass experienced bloating or decay stages. Although the beetles were not significantly present in the fresh phase, in the final phase of decomposition, specimens associated with the carcass were 221 representing all species recorded in this study, except Oxelytrum discicolle (Silphidae), which was not observed during the last 3 days. Beetles of the family Cleridae (163; 30.6%) and Dermestidae (142; 26.6%) were observed around the second day of exposure of the carcass and reached greater abundance when the pig’s remains were dry. The families Cleridae and Dermestidae were present in all phases of decomposition. In relation to Cleridae, Necrobia rufipes (DeGerr, 1775) (86; 1.7%), Necrobia ruficollis (Fabricius, 1775) (49; 1.0%) and Necrobia sp. (28; 0.6%) were the most abundant (Table 1).
According to Bala et al.,61 Necrobia rufipes and Dermestes maculatus represents the most collected species in forensic experiments, which were also recorded in this study. Carvalho et al.,25 describes that these Coleoptera are easily recognized by the metal-colored body, covered with bristles and antennas of four joints. The observations regarding the families of beetles reported in this study corroborate the reports11 who states that members of the Cleridae family feed on larvae and fat attached to dry bones. As for the Dermestidae family, also observed in all phases, especially coliquative, fermentative and skeletonization, Dermestes maculatus (DeGeer, 1774) (86; 1.7%) and Dermestes sp. (56; 1.1%) were identified (Table 1). Regarding the other families, in a study by Carvalho and collaborators55 adults of Hysteridae only colonized the carcass on the twelfth day, while in this work they were present from the second day. The same author reports that the representatives of Staphylinidae were in the carcass on the thirteenth day, final stage of putrefaction. On the other hand, in this study the Staphylinidae were found more frequently on the ninth and tenth days. In general, Silphidae are insects that live around the bodies of animals, although some species live on fungi and anthills.62 According26 these insects are necrophagous in the larval phase and predators as adults.
In this study, collected Silphidae representatives were Oxelytrum cayennense (Stürns, 1826) and O. discicolle (Brullé, 1840). Wolff et al.,63 captured adults of Oxelytrum spp. during the active, advanced and dry decomposition phases, while in the present study Oxelytrum discicolle was already present during the bloat phase, and it was the only Coleoptera not observed in the final dry phase. Oxelytrum cayennense colonized the carcass from the emphysematous stage to skeletonization. The results observed in this study are consistent with those found by Ururahy-Rodrigues and col.62 who observed this predator species in the second day of postmortem interval. On the seventh day of the present experiment, a dead specimen of this species was found near the carcass. Ants from the Solenopsis genus carried the specimen when they were intercepted and the beetle was collected, which was part of the final specimen count. Tera, besouros (Coleoptera), vespas (Hymenoptera), baratas (Blattaria) e ácaros. Insects known as ants make up the Formicidae family, and are found in all environments of the world, except cold regions and on water.64 More than 13,505 valid species are currently known, distributed in 334 genera and 17 subfamilies.65
In terms of faunistic succession, Hymenoptera are the third most numerous insect orders present in carcasses, and Formicidae is the most representative family.5 Hymenoptera, in this study represented by the Formicidae family, were classified as the third group of greatest forensic interest. During the experiment period, a total of 1.628 specimens, distributed in five subfamilies (Myrmicinae, Formicinae, Paraponerinae, Ponerinae, Ectatominae) and nine tribes (Solenopsidini, Pheidolini, Crematogastrini, Camponotini, Lasiini, Paraponerini, Ponerini, Odontomachini, Attini, Ectotommini). A total of 16 taxa, distributed in eleven genera, were identified: Solenopsis, Camponotus, Paraponera, Dinoponera, Paratrechina, Atta, Odontomachus, Acromyrmex, Ectatomma, Crematogaster and Pheidole. According to Campobasso et al.,5 ants are biological agents typically observed shortly after the animal's death and during all phases of decomposition. The subfamily that presented the highest richness was myrmicinae (594; 36.5%), with seven morpho-species and five genera (Solenopsis, Pheidole, Crematogaster, Atta, Acromyrmex).
The genera that presented the highest number of morpho-species were, in order: Ectatomma (Ectatoma edentatum (Roger, 1863), E. opaciventre (Roger, 1861), (Ectatoma spp.), followed by Solenopsis (Solenopsis invicta (Buren, 1972) and S. saevissima (Smith, 1855)), Camponotus (Camponotus rufipes (Fabricius, 1775), C. melanoticus (Emery, 1894)), Dinoponera (Dinoponera gigantea (Perty, 1833), D. mutica (Emery, 1901)), Acromyrmex (Acromyrmex subterraneus (Forel, 1893), Acromyrmex spp.), Pheidole spp; Crematogaster spp; Paraponera (Paraponera clavata (Fabricius, 1775)), Odontomachus spp; Paratrechina (Paratrechina longicornis (Latreille, 1802)) and Atta spp. (Table 1). The ants of the Atta genus “Saúvas” occur in Brazil in 10 species and three subspecies.66 The genus Acromyrmex “Quenquéns” has 63 species, of which 28 have been found in Brazil.67 It was detected the occurrence of leaf-cutting ants of the taxa Atta spp. (34; 0.7%), Acromyrmex subterraneus (38; 0.8%) and Acromyrmex spp. (76; 1.5%) in the study area (Table 1). In Mato Grosso do Sul68 showed that ants may be present in all stages of decomposition, including the genera isolated in this work. In Mexico69 found ants of the Atta genus actively foraging in the wounds of mammals, describing the action of ants as opportunistic agents. It was observed during the experiment that some individuals of Acromyrmex subterraneus and Camponotus rufipes transported small pieces of cadaveric material in their mandibles, as well as larvae of dipterans.
It was observed that the genus Odontomachus, a genus of carnivorous ants, presented the same habit. The striking feature of this species, known as trap-ants, is the bearing of a pair of large straight jaws capable of opening up to 180 degrees. The importance of these insects in the process of mammalian decomposition was recorded70 who evaluated the carcass of Mus musculus in the early stages of decomposition. Ants from the Camponotini and Crematogastrini tribes were found, mainly in the nasal, oral and auricular mucosae. Fonseca et al.,71 recorded the subfamilies: Myrmicinae (Crematogaster spp.) and Formicinae (Camponotus melanoticus) as dominant in the carcass of Rattus norvegicus. The records of Chen et al.,72 in Malaysia show the importance of these insects in forensic studies when using monkeys as indicators of decomposition. These researchers, in their casuistic, found information that ant species could act as geographic indicators, highlighting the species Pheidole longipes and Paratrechina longicornis, distributed in different habitats. Paratrechina longicornis, popularly known as the crazy-ant by its semicircular trail, was recorded in this study (92; 1.8%), as well as Pheidole, known as big-headed ant (87; 1.7%) (Table 1). Specimens of the taxon Crematogaster, arboreal ants of the Myrmicinae (Crematogastrini) subfamily, were identified from the 2nd day of exposure.
In the gas phase, they were found feeding on the opened wounds, as well as the blood and tissue close to the exposed lesions. The specimens appeared around the mouth, tongue, eyes on the abdomen and external genitalia. Another representative of Formicidae, Paraponera clavata, known in the central region of Brazil as "Tucandira", presents primitive characteristics and painful bites.73 Ramon et al.,74 in their observations, report that this pungent behavior can potentially leave injuries in the decaying body, which can provide valuable evidence for the investigation. In this sense, ants of the order Hymenoptera interact on decomposition both by its predator action on eggs, pupae, larvae and other adult arthropods and by the damage they can cause to the bones, tissues, exudate, skin and attachments of the corpse.58 Known in Brazil as "lavapés" and around the world like fire ants (Myrmicinae: Solenopsidini) are aggressive insects whose bites can cause allergic reactions when disturbed or postmortem lacerations to dead animals.14 Catts et al.,9 emphasize that this genus can help in PMI estimation by calculating the time required to establish a colony or nest on the body associated with the decomposition process. Representatives of the genus Ectatomma were scattered over the carcass and around them, feeding on the blood from the openings caused by the gaseous phase. In this study, we identified: Ectatomma edentatum (123; 2.5%), Ectatomma opaciventre (110; 2.2%) and Ectatomma spp. (74; 1.5%) (Table 1). It is possible to verify an association between some species of ants and fungi, due to the environment explored by these insects.
Rodrigues et al.,75 found, in Paratrechina longicornis, fungal species of the Ascomycota and Zygomicota phylla that are part of the microbiota of these ants. Zarzuela et al.,76 found several fungi belonging to the Aspergillus, Epicoccum, Fusarium, Neurospora, Niger and Rhizopus genera associated with various ants in the southeast of Brazil, including P. Longicornis. Zettler et al.,77 found, in Solenopsis invicta and P. Longicornis, specimens of the genera Absidia and Penicillium. Some of these genera of fungi were isolated in this study, showing that ants can act as dispersers of saprophytic and pathogenic fungi. The results obtained in this study are added to other records to form a basis for future investigations regarding the ecology of Formicidae in the process of cadaveric decomposition. According78 there are 5.1million fungal species on our planet, in the most varied natural sites, assuming important roles in pathogenic processes and in the degradation and recycling of organic matter. Fungi can be a useful tool in identifying the postmortem interval, providing useful evidence for solving cases79 when forensic entomology cannot be applied.80 There are controversies among researchers regarding forensic mycology and the use of fungi in the identification of cadaveric processes. Menezes et al.,81 argue that there is still no forensic tool established for the determination of the postmortem interval and that the use of fungi is still premature.
França,4 report that among the most complex problems is the determination of the approximate time of death in the absence of chronological elements, such as those obtained from necrofauna, capable of providing a sequential characterization of cadaveric phenomena. In recent decades, studies describing the use of fungi as a forensic tool have been published referring to the countries: Japan,80,81 United States,82 China,83 Germany,84 Argentina,70 Romania85 and Brazil.40,86‒88 In this study, 223 fungal specimens (Figure 3), distributed in three phylla, twelve orders, 21 genera and 35 species, were identified. Phyllum Ascomycota was the most representative (179; 80.3%), followed by Zygomycota (33, 14.8%) and Basidiomycota (11; 4.9%) (Table 2). In China83 found Ascomycota the most dominant phyllum, followed by Basidiomycota, Zygomycota and Chrytridiomycota. Most isolated fungi were filamentous (190; 85.2%) as opposed to yeasts (33; 14.8%). Among filamentous fungi identified, the following taxonomic distribution was observed: 99 CFUs from Eurotiales (44.4%), represented by genera Aspergillus, Penicillium, Talaromyces and Paecilomyces; 33 CFU’s from Mucorales (14.8%)-Mucor, Rhyzopus, Circinella and Mycocladus; 18 CFU’s from Hypocreales (8.1%)-Fusarium and Trichoderma; 12 CFUs from Capnodiales (5.4%)-Cladosporium; 11 CFU’s from Sordiales (4.9%)-Chrysonilia; 7 CFU’s from Pleosporales (3.1%) – Alternaria and Pithomyces; 3 CFUs from Helotiales (1.3%)–Bothrytis and, finally, 2 CFU’s from Trichosphaeriales (0.9%–Nigrospora (Table 2). In respect to yeasts, the following taxonomic distribution was observed: 22 CFUs from Saccharomycetales (9.9%), represented by the genera Candida and Pichia; 8 CFUs from Sporodiales (3.6%)–Rhodotorula and 3 CFU’s from Tremellales, represented by the Trichosporon genus (Table 2).
Fungi (N) Total: 223 fungi |
Phases – CFU |
|
|
||||||
Phyllum |
Order |
Isolated species |
I |
II |
III |
IV |
V |
N |
% |
Ascomycota |
Capnodiales |
Cladosporium herbarum |
0 |
1 |
3 |
0 |
2 |
6 |
2,7 |
Ascomycota |
Capnodiales |
Cladosporium cladosporioides |
0 |
0 |
4 |
0 |
2 |
6 |
2,7 |
Ascomycota |
Eurotiales |
Aspergillus aculeatus |
2 |
1 |
1 |
0 |
2 |
6 |
2,7 |
Ascomycota |
Eurotiales |
Aspergillus carbonarius |
0 |
3 |
0 |
0 |
1 |
4 |
1,8 |
Ascomycota |
Eurotiales |
Aspergillus flavus |
4 |
2 |
3 |
0 |
3 |
12 |
5,4 |
Ascomycota |
Eurotiales |
Aspergillus clavatus |
2 |
0 |
3 |
0 |
2 |
7 |
3,1 |
Ascomycota |
Eurotiales |
Aspergillus niger |
3 |
3 |
4 |
0 |
0 |
10 |
4,5 |
Ascomycota |
Eurotiales |
Aspergillus ochraceus |
0 |
1 |
1 |
0 |
2 |
4 |
1,8 |
Ascomycota |
Eurotiales |
Aspergillus terreus |
3 |
4 |
7 |
0 |
2 |
16 |
7,2 |
Ascomycota |
Eurotiales |
Penicillium citrinum |
2 |
2 |
3 |
2 |
3 |
12 |
5,4 |
Ascomycota |
Eurotiales |
Penicillium expansum |
0 |
5 |
4 |
2 |
3 |
14 |
6,3 |
Ascomycota |
Eurotiales |
Penicillium griseofulvum |
1 |
3 |
0 |
0 |
1 |
5 |
2,2 |
Ascomycota |
Eurotiales |
Talaromyces rugulosus |
0 |
0 |
3 |
1 |
0 |
4 |
1,8 |
Ascomycota |
Eurotiales |
Paecilomyces viride |
0 |
2 |
2 |
1 |
0 |
5 |
2,2 |
Ascomycota |
Helotiales |
Bothrytis cineria |
0 |
0 |
2 |
0 |
1 |
3 |
1,3 |
Ascomycota |
Hypocreales |
Fusarium graminearum |
1 |
0 |
0 |
1 |
1 |
3 |
1,3 |
Ascomycota |
Hypocreales |
Fusarium oxysporum |
1 |
0 |
3 |
0 |
2 |
6 |
2,7 |
Ascomycota |
Hypocreales |
Trichoderma harzianum |
0 |
0 |
1 |
0 |
3 |
4 |
1,8 |
Ascomycota |
Hypocreales |
Trichoderma viride |
0 |
0 |
1 |
2 |
2 |
5 |
2,2 |
Ascomycota |
Pleosporales |
Alternaria alternata |
1 |
0 |
0 |
1 |
2 |
4 |
1,8 |
Ascomycota |
Pleosporales |
Pithomyces chartarum |
1 |
2 |
0 |
0 |
0 |
3 |
1,3 |
Ascomycota |
Saccharomycetales |
Candida kefyr |
2 |
1 |
0 |
1 |
2 |
6 |
2,7 |
Ascomycota |
Saccharomycetales |
Candida krusei |
1 |
2 |
1 |
1 |
1 |
6 |
2,7 |
Ascomycota |
Saccharomycetales |
Candida tropicalis |
1 |
1 |
0 |
1 |
1 |
4 |
1,8 |
Ascomycota |
Saccharomycetales |
Pichia anômala |
1 |
2 |
2 |
0 |
1 |
6 |
2,7 |
Ascomycota |
Sordoriales |
Chrysonilia sitophila |
3 |
3 |
1 |
2 |
2 |
11 |
4,9 |
Ascomycota |
Trichosphaeriales |
Nigrospora nigri |
0 |
0 |
0 |
0 |
2 |
2 |
0,9 |
Ascomycota |
Xyraliales |
Pestalotiopsis microspora |
0 |
0 |
2 |
1 |
2 |
5 |
2,2 |
Zigomycota |
Mucorales |
Circinella muscae |
1 |
1 |
0 |
0 |
0 |
2 |
0,9 |
Zigomycota |
Mucorales |
Mucor hiemalis |
0 |
2 |
0 |
0 |
1 |
3 |
1,3 |
Zigomycota |
Mucorales |
Mucor racemosus |
1 |
1 |
3 |
1 |
1 |
7 |
3,1 |
Zigomycota |
Mucorales |
Mycocladus ramosus |
0 |
1 |
0 |
0 |
1 |
2 |
0,9 |
Zigomycota |
Mucorales |
Rhizopus oryzae |
2 |
2 |
3 |
2 |
3 |
12 |
5,4 |
Zigomycota |
Mucorales |
Rhizopus stolonifer |
2 |
2 |
0 |
0 |
3 |
7 |
3,1 |
Basidiomycota |
Sporodiales |
Rhodotorula mucilaginosa |
2 |
2 |
1 |
1 |
2 |
8 |
3,6 |
Basidiomycota |
Tremellales |
Trichosporon spp. |
2 |
0 |
0 |
0 |
1 |
3 |
1,3 |
Total |
- |
- |
39 |
49 |
58 |
20 |
57 |
223 |
100 |
Percentage % |
- |
- |
17,5 |
22,0 |
26,0 |
9,0 |
25,6 |
100 |
- |
Table 2 Distribution of fungal specimens collected on Sus scrofa (Linnaeus) carcass in vale do Jamacá, Chapada dos Guimarães, Mato Grosso, Brazil (2018)
I, Fresh/Chromatic phase; II, Putrefaction/Bloat Phase; III, Active decay/Dark putrefaction phase; IV, Advanced decay/Fermentation phase; V, Skeletonization/Dry phase
As the most abundant species of fungi isolated from the Sus scrofa carcass, Aspergillus terreus (16; 7.2%); Penicilium expansum (13, 5.8%); while Aspergillus flavus, Penicillium citrinum and Rhizopus oryzae presented the same number (12, 5.4%). Aspergilli, belonging to phyllum Ascomycota, were most commonly isolated in this study, especially Aspergillus terreus, which are highlighted among the other seven identified species (Table 2). These fungi are recognized as efficient saprophites, being commonly found in the soil.89 They can also be found indoors, where they release spores, easily transported by air, called anemophile entities.90 In fact, the dissemination by anemochory is one of the main forms of propagation of many fungi, and of extreme efficiency.91,92 In Rio Grande do Sul, Brazil86 reported the isolation of mycelia-forming fungi of the genus Aspergillus, Penicillium, Fusarium, Cladosporium and Alternaria as members of swine microbiota. In yeasts, among the isolates, the most prominent was the species Rhodotorula mucilaginosa (8; 3.6%) followed by the species Candida kefyr, C. krusei and Pichia anomala (6; 2.7%) respectively. Sequentially, Candida tropicalis (4; 1.8%) and Trichosporon spp. (3; 1.3%) were isolated (Table 2). In older reports93 identified yeasts of the genus Candida associated with the swine intestine, where the species C. tropicalis and C. Krusei, were highlighted. That could explain the isolation of these species from oral and anal mucosa in the present study (Figure 4).
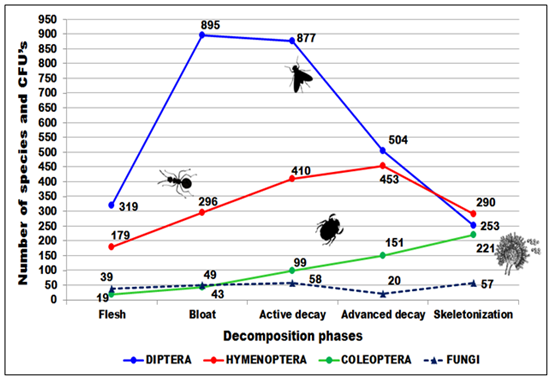
Figure 4 Number of fungi collected on Sus scrofa (Linneus) experimental model in vale do Jamacá, Chapada dos Guimarães, Mato Grosso, Brazil (2018).
The presence of yeasts of the genera Candida, Rhodotorula, Pichia and Trichosporon isolated from the carcass, was consistent with the results presented by Carregaro et al.,86 which demonstrated the coexistence of these species with specimens of S. scrofa in southern Brazil. Of the 119 samples sent to laboratory analyses, the number of CFU’s detected was, by anatomic site: oral mucosa (17; 7.6%), nasal mucosa (34; 15.2%), ear canal (15; 6.7%), anal and genital mucosa (39; 17.5% each) and skin (50; 22.4%). In the skeletonization phase, it was easy to perceive the thanatological evolution and reduction of the anatomical sites, as there was no longer the presence of mucous membranes, samples were collected on the dry bones (29, 13.0%) and skin (50; 22.4%), exposed (rib, femur and skull) during the last four days (Figure 4). In relation to the phase of decomposition, there were more fungi isolated, in decreasing order, on the third phase/ initial putrefaction (58; 26.0%), fifth phase/period dry/skeletonization (57; 25.6%), second phase/emphysematous (49; 22.0%), first phase/fresh (39; 17.5%) fermentative/colliquative phase (20; 9.0%) (Table 2). It could be hypothesized that the feeding of insects in the island of cadaveric decomposition, fungal particles are ingested, contributing to less fungal isolation.
Rohlfs et al.,94 they recall that many resistant spore-releasing fungi release attractive secondary metabolites, allowing their ingestion by arthropods, thus becoming an efficient mean of dispersion by invertebrates. From the moment the vital signs cease, the cadaveric phenomena begin, where the microbiota initiates the phenomena of bodily disintegration. This situation is similar to clinical practice when comparing residual microbiota in healthy hosts. In that case, they may trigger physiological shifts: immunodeficiency, use of immunosuppressive drugs, cancer and infections, where a high isolation of microorganisms is expected due to immunologic weakness.95 In this sense, higher frequencies in the isolation of yeasts were observed in the fresh, emphysematous and colliquative periods, where this appearance may be linked to the decrease in the action of bacteria. Therefore, the fungal microbiota of the mucous membranes can perpetuate its growth, since many species constitute mucosae microbiota.46
Vanrell56 indicates that this fact may be linked to the process of degradation suffered by the corpse, when the bodily barriers begin to deteriorate and there is communication between the anatomical parts, facilitating the access of microbiota agents to other sites of the body. Mycologist’s researchers40 found fungi on human corpses in northeast Brazil. The samples were collected from the mucous membranes, skin, clothes and hair. They also observed the genera Aspergillus, Penicillium and Candida in their study. Goebel et al.,87 in the same sense, isolated the filamentous fungi Penicillium spp. and yeasts from Candida genus from pig carcasses in southern Brazil. In their study88 found the filamentous genera: Acremonium, Aspergillus, Cladosporium, Curvularia, Mucor, Scedosporium. Among yeasts, seven species were identified: Arthrographis spp, Rhodotorula spp., and we highlight the presence of Candida guilliermondii, Candida krusei, Candida lipolytica, Candida tropicalis and Candida zeylanoides. Around the world, Schwarz et al.,84 investigated the presence of fungi in autopsies in Germany and observed 24 species in skin samples, from 11 genera: Aspergillus, Candida, Debaryomyces, Helicostylum, Lichtheimia, Mucor, Penicillium, Pseudogymnoascus, Rhizopus, Scopulariopsis and Yarrowia. Chinese researchers83 found genera Mikaria, Aspergillus, Amorphotheca, Ophiocordyceps and Alternaria during the decomposition process. In Argentina79 found fungi on two cadavers and identified the genera Arthrinium, Aspergillus, Candida, Cladosporium, Chrysosporium and Scopulariopsis. Representatives from the Aspergillus and Penicillium genera are known as anemophiles and grow easily on any organic substrate.17,90
The following fungi from Aspergillaceae family were isolated in all phases, especially on the bloat phase: Aspergillus (19; 8.5%), Penicillium (7; 3.1%) and Talaromyces (3; 1.3%) (Table 2). After detecting fungi from the order Mucorales and Hypocreales, which include the genera Trichoderma and Fusarium, it was clear that the cadaveric ecosystem can host other decomposer species. In this study, it could be observed a succession through the decomposition process between the orders Capnodiales, Eurotiales, Helotiales, Hypocreales, Pleosporales, Saccharomycetales, Sordoriales, Trichosphaeriales, Xyraliales, Mucorales, Sporodiales and Tremellales (Table 2). The ecological succession of these species of fungi demonstrates the interaction of these organisms with other groups of necrophilic agents, showing that this group of organisms can be used as an indicator of time of death. During the final phase, skeletonization period (15th to 17th day), there was an increase in the number of isolations (57 CFU’s) (Figure 3). In that context, the carcass is not as exuberant in terms of organic matter content, which contrasts with the previous phase, dark putrefaction, where fewer CFU’s were detected.
The results of this research are consistent with the results of Japanese researchers80,96 which report similar conditions in fungal development. Menezes et al.,97 reinforce this exploratory action, emphasizing that when analyzing fungal growth, the different climatic conditions in which these agents are found have to be taken into account. Furthermore, additional experimental studies should be conducted, due to the growth rates and fungal patterns. Hösükler et al.,85 emphasize that, just as insects show a pattern of postmortem succession, fungi need to be studied in order to verify whether they also exhibit a successive pattern that can assist in the determination of PMI. In view of the analyses and observations made during this study, the variety of isolated species should be taken into consideration. Lacaz et al.,41 emphasize that fungal agents can be opportunistic, colonizing preexisting cavities. Their conidia become easy to spread and may be contamination agents, demanding safety standards.84 It is important to observe their ecological and pathological characteristics, because these entities assume epidemiological relevance to professionals during autopsy, exposing them to these organisms implicated in human mycoses and pulmonary infections and even severe systemic manifestations.
The use of insects as parameters for the identification of crimes in the forensic scenario is well established mainly using Diptera and Coleoptera as traces in the elucidation of crimes. Fungi, however, were considered for a long time only as agents of biological degradation, and their use was underestimated. Nowadays, these eukaryotes have shown an important role in human daily life, in addition to their involvement in human and plant diseases, as well as food control. The chronology of postmortem events presents several factors that exhibit case by case variation. This study has shown diverse findings. The determination of the time of death is a difficult diagnosis and often its factors become difficult to obtain. Usually associated with rot and organic decay, fungi have been neglected as to their potential and resourceful organism that could help mankind with some of its greatest problems.
This study opens a new perspective where fungi play a relevant role in forensic science. The report of these findings already allows drawing a horizon for the subject, where careful analysis of the fungal elements can be used as parameters for PMI estimation. This paper presents an initial step in the establishment of fungal specimens in cadaveric phenomena, requiring research with a larger sample number to better characterize and identify the mycological findings. In the same sense, in order to establish the real role of these entities, the gaps in studies involving these microorganisms as a tool in technico-scientific and medico-legal investigations must be fulfilled.
Diniz Pereira Leite Júnior was the lead researcher-in-chief and performed the study design. D.P.L.J. and Elisangela Santana de Oliveira Dantas conducted the study, performed entomological specimen collection and identification. D.P.L.J. and Claudete Rodrigues de Paula isolated and identified fungal specimens. D.P.L.J. wrote the manuscript and edited figures/tables. Heitor Simões Dutra Corrêa performed the English translation of the manuscript. All authors performed data analysis, as well as manuscript assessment, revision and approval.
For thank financial support provided by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Conselho Nacional de Pesquisa (CNPq)-Brazil.
The author declares the absence of conflicting interests in study design; data collection, analysis and interpretation; manuscript writing; decision to publish the results.

©2019 Júnior, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.