International Journal of
eISSN: 2381-1803


Review Article Volume 15 Issue 5
1 Department of Genetics and Morphology, University of Brasília, Brazil
2 Department of Mechanical Engineering, University of Brasília, Brazil
3 Department of Mechanical Engineering, University of Brasília, Brazil
Correspondence: Joabe Lima Araújo, Postgraduate Program in Nanoscience and Nanobiotechnology, Department of Genetics and Morphology, Institute of Biological Sciences, Darcy Ribeiro University Campus, University of Brasília, Brasília-DF 70910-900, Brazil
Received: September 04, 2022 | Published: September 27, 2022
Citation: Vieira JA, Araújo JL, Silva MS, et al. Advantages of the use of polymeric nanoparticles in the treatment of breast cancer: a systematic review. Int J Complement Alt Med. 2022;15(5):266-272. DOI: 10.15406/ijcam.2022.15.00618
Introduction: Breast cancer is responsible for a fifth of the total number of patients diagnosed with malignant neoplasms worldwide. Given the limitations of conventional treatments, studies are advancing to develop new therapies with more specificity and fewer side effects. Thus, the use of polymeric nanoformulations for the delivery of chemotherapeutic agents has been developed for biomedical applications.
Objectives: therefore, the study aimed to investigate the advantages and effectiveness of the use of polymeric nanoformulations in drug delivery in relation to conventional therapies in the treatment of breast cancer.
Method: bibliographic searches were performed using specific search strings, totaling 12 articles collected.
Result: the results showed the effectiveness of the strategy of using polymeric nanoparticles for drug delivery, highlighting the increase in cellular uptake and sustained intracellular retention of drugs incorporated into the nanoparticles. It was evidenced that in addition to the increased efficacy, the safety and delivery of the drug improved.
Conclusion: this strategy improved the performance of the drug, having a higher concentration of the active ingredient in the tissues of cancer patients, in addition to increasing the uptake and cellular concentration of the drug, as well as sustaining its intracellular retention, which increases the effectiveness of the treatment, due to its prolongation linked to the slow release and in smaller amounts of the drug.
Keywords: nanoparticles, drug delivery system, drug utilization, breast cancer
Breast cancer represents a fifth of the total number of patients diagnosed with malignant neoplasms worldwide.1 This disease is characterized by the uncontrolled division of abnormal cells by mitosis in the breast, resulting in a tumor. This tumor, in turn, can present itself in different ways depending on the characteristic of breast carcinoma.2 After diagnosis, according to the stage and type of tumor, the patient undergoes treatment that may include surgery, radiotherapy, chemotherapy, and immunotherapy, which are conventional therapies. However, these treatments have reduced efficacy and can cause adverse effects that can lead to treatment discontinuation due to debilitation of the patient and risk of life.3 This is due to chemotherapeutic agents that cannot differentiate cancer cells from normal cells, leading to systemic toxicity causing cell death of both normal and cancerous cells.4
In addition, the permeation of these anti-cancer agents in tumor cells is very limited, as they have less distribution and rapidly elimination, which results in greater administration of the drug to the patient, causing undesirable toxicity and discomfort. It also decreases its effectiveness, as surviving cells become increasingly resistant to treatment.5
Thus, studies have presented the use of nanoformulations for the delivery ofchemotherapeutic agents, in which nanoparticles (NPs) perform this distribution in a controlled and more effective way.6 This distribution of drugs through NPs allows drug protection and effectiveness in delivering the desired concentration to the target tumor, increasing drug efficacy and minimizing side effects, as well as reducing toxicity in non-cancerous cells.4
The most common nanoformulations in studies of improved drug synthesis according to Urrejola et al.7 are dendrimers, polymeric-NPs (NPsP), liposomes, nanoemulsions and Polymeric Micelles (PM). NPsP have a diameter between 10 and 1000 nm, in which the drug is encapsulated, dissolved, adsorbed or dispersed in the polymer matrix.8,9 According to Zolnik et al. [10], the main characteristic of NPsP is to promote the prolonged action of the bioactive compound as a result of its sustained release and for the protection of the compound from enzymatic degradation.
There are two types of NPsP for this purpose, namely nanospheres and nanocapsules. Nanospheres are spherical matrix systems, which have the active agent dispersed in a polymeric matrix with a homogeneous characteristic. Nanocapsules, on the other hand, are vesicular systems that surround the agent within a cavity surrounded by a polymeric shell, which controls its release depending on its nature. Both types of NPs have different properties in terms of how to release their active ingredient. The selection will depend on the manufacturing method, chemical and physical properties and characteristics of the drug to be used.7
Breast cancer is the most prevalent cancer in the world according to data from the World Health Organization.11 Since the mortality rate from breast cancer has increased dramatically in recent years, a more effective anti-cancer treatment with fewer side effects to the patient is needed. Thus, this systematic review study aimed to investigate the advantages and effectiveness of the use of polymeric nanoformulations in drug delivery compared to conventional therapies in the treatment of breast cancer.
Type of study
This study is a systematic review and was developed according to the following steps: topic selection, establishment of study eligibility criteria, selection of articles from the search strings in the databases, analysis of the found articles, screening of articles in two stages, interpretation of results and organization of data layout, according to the protocol of Luz et al.12 with some modifications.
Eligibility criteria
The criteria adopted in this systematic review followed the approach of the acronym PICOS (Population, Intervention, Comparison, Outcome and Study Design).13 In which rats, mice, and humans, of both sexes, of different ethnicities, diagnosed with breast cancer, seeking alternative medical treatment (P); with drugs incorporated into NPsP (I); compared to treatment with free drugs, chemotherapy, radiotherapy, immunotherapy, among others (C); which have cytotoxic activity and efficacy at the end of treatment, becoming an alternative strategy in the treatment of breast cancer, with less consequences for the patient (O).
This study considered review articles and original peer-reviewed articles, published in English (American and British), on NPsP used for drug delivery in the treatment of breast cancer compared to the use of free drugs. The results were organized into sub-themes for a better understanding of the subjects covered in this research, considering a period of searches in the databases of the last 10years, between 2011 to 2021.
Data screening items
The bibliographic searches were performed on January 15, 2022, in the respective databases: PubMed/MEDLINE, Embase and Scopus.
Selection of studies and data collection process
The articles used in this study were selected after bibliographic searches in the databases. Thus, the articles were screened in two stages, and in the first stage, four independently reviewers evaluated all the articlesconsidering the eligibility criteria established in this study. Then, in the second stage, the full texts of each article were readand evaluated. At the end, the articles that presented relevance to the predefined themes and/or sub-themes were selected. Subsequently, the data obtained was organized in a "Results and Discussion" section written by the seven reviewers of this study strictly following the PRISMA 2020 verification protocol for a systematic review study.14,15
Analysis of results
After the bibliographic searches in the databases, the data of the articles found were tabulated in an Excel spreadsheet (Microsoft Excel tool (2007)) for analyses. All the extracted files were computed in the Mendeley Desktop 2.61.1 software, which was used to remove duplicates by automation. In addition to these tools, was used the freely available VOSviewer 1.6.8 software (https://www.vosviewer.com/) to assess citation relationship networks, bibliographic coupling and co-citations related to the descriptors of this study.
Risk of bias and quality of evidence
The studies collected in this systematic review showed methodological heterogeneity in the conduct of each research. In which, they ranged from pre-clinical studies to randomized and non-randomized clinical trials. Thus, was evaluated the quality of the studies, in which twoindependent reviewers assessed the risk of bias of the articles included through the application of a questionnaire and submitted to the RoB 2.0 tool (Revised Cochrane risk-of-bias tool for randomized trials),16 which evaluated the quality and bias of randomized clinical trials and the ROBINS-I tool (Risk Of Bias In Non-randomised Studies - of Interventions)17 for non-randomized clinical trials. In addition, was used the "GENERIC" tool to assess the quality of preclinical studies (in vitro, ex vivo and in vivo). It is worth noting that all these tools are part of the Robvis (Risk-of-bias VISualization) software package.18 In case of disagreements between the first two reviewers, a third reviewer was requested.
The Risk of Bias Tools are based on 05 domains, namely: 1) Bias resulting from the randomization process; 2) Bias due to deviations from intended interventions; 3) Bias due to lack of results data; 4) Bias in outcome measurement; and 5) Bias in the selection of the reported study.19
Systematic review record
This systematic review study received deposit approval in the Prospective Register of Systematic Reviews (PROSPERO) under No. CRD42022315638, as can be accessed at the link: (https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022315638). This platform is a systematic review study registry base, in which the research registry promotes transparency and eligibility in the execution of the study. In addition, it aims to document systematic reviews already developed or in progress, avoiding the simultaneous execution of research on the same topic by different groups, which prevents and monitors plagiarism.
The scientific survey was carried out on January 15, 2022, in the PubMed/MEDLINE, Embase and Scopus databases using descriptors related to the PICO acronym mentioned in the subsection “Eligibility Criteria” in the topic Method of this study, in which the strings were obtained specific search queries for each database, as available in Table 1.
Bancos de dados |
Strings |
PubMed/MEDLINE |
(((((“Humans”[Mesh]) AND “Adult”[Mesh]) AND “Neoplasms”[Mesh]) AND “Drug Delivery Systems”[Mesh]) AND “Polymers”[Mesh]) AND “Nanoparticles”[Mesh] |
Embase |
‘human’/exp AND ‘adult’/exp AND ‘breast tumor’/exp AND ‘drug delivery system’/exp AND (‘nanoparticle’/exp AND ‘polymer’/exp OR ‘polymer nanoparticle’/exp) |
Scopus |
( TITLE-ABS-KEY ( “homo sapiens*” OR man* OR “modern man*” OR human* ) AND TITLE-ABS-KEY ( “breast neoplasm*” OR “breast tumor*” OR “breast cancer*” ) AND TITLE-ABS-KEY ( pharmaceutical* OR “pharmaceutical preparation*” OR ( preparation* AND pharmaceutical* ) OR drug* ) AND TITLE-ABS-KEY ( ( nanoparticle* AND polymeric* ) OR “polymeric nanoparticle*” OR “nanosized particle polymeric*” ) AND TITLE-ABS-KEY ( therap* OR drug* OR “drug therap*” OR “drug harmacother*” OR harmacother* OR harmacotherapy* ) AND TITLE-ABS-KEY ( adult* ) ) |
Table 1 Strings used for bibliographic searches in databases
*Microsoft Word (2007). Source: Author (2022).
Selection of studies and data collection process
The bibliographic searches in the databases were successfully carried out, with a total of 52 scientific articles being found, in which 25 studies remained after removing the duplicates, which were performed by automation using the Mendeley Desktop 2.61.1 software. Thus, was obtained a total of 11 scientific articles in the Embase database, 8 in PubMed/MEDLINE and 6 in Scopus (Figure 1). After analyzing the inclusion and exclusion of articles based on the eligibility criteria established in this study, 12 articles were selected for this systematic review (Table 2). Of these, 7 are pre-clinical in vitro and/or in vivo studies, 4 clinical studies and 1 literature review studies, as available in Table 2. It is worth mentioning that of the 52 articles found, only 1 article presented a duplicate in the Scopus database and 26 articles in the Embase database from which they were removed.
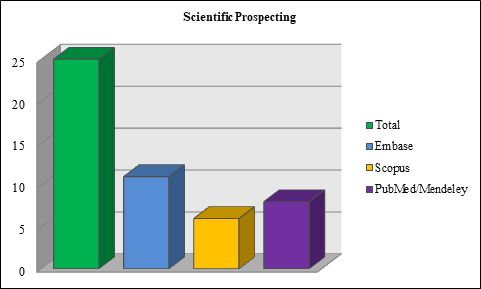
Figure 1 Number of articles found in the databases.
*Analysis in Microsoft Excel (2007). Source: Own authorship (2022).
Therefore, the criteria established in the PICO acronym were essential for the selection of articles and removal of terms in the DECs and MeSh bases, for the construction of specific search strings that were applied to extract the data in each bibliographic database, bringing greater reliability and basis for this study. In addition to checking the PRISMA 2020 checklist for systematic review study that is available to readers as supplementary material (Table S1) and the PRISMA 2020 Diagram presented in Figure 2.
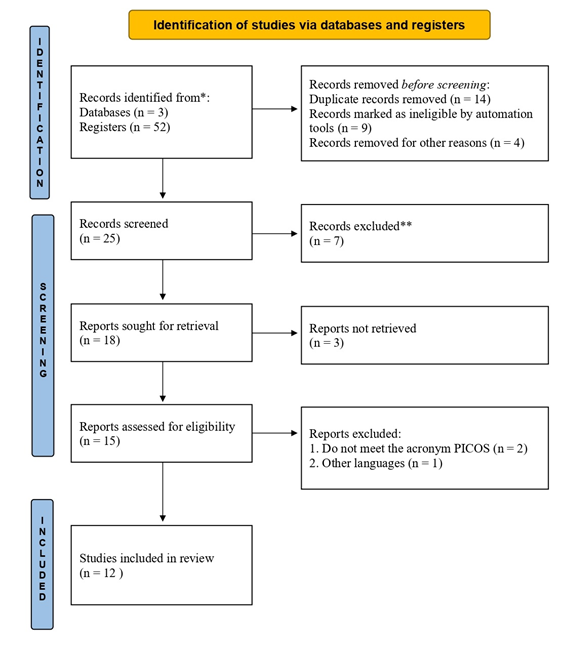
Figure 2 PRISMA 2020 diagram: methods and filters adopted in bibliographic searches and selection criteria according to the PRISMA 2020 protocol.15
We also analyzed the keyword network of the search findings (n = 25) in the VOSviewer 1.6.8 software, as shown in Figure 3. The different sizes of the nodes represent the number of times each word is cited, the links refer to co-citations. That is, if the same word is cited by more than one article, the node will be larger and if it has co-citations, it will have links with other keywords. We passed as a restriction to the software that each keyword would need to be cited at least twice in the article, thus we obtained 174 nodes. This analysis was performed after removing duplicates using the Mendeley Desktop 2.61.1 software, in which we can observe that the words and links in green color are the closest to the theme of this study, represented by the words "nanoencapsulation", "breast cancer ", "human", "in vitro study", "adult", "animal cell", "micelle" and "drug formulation".
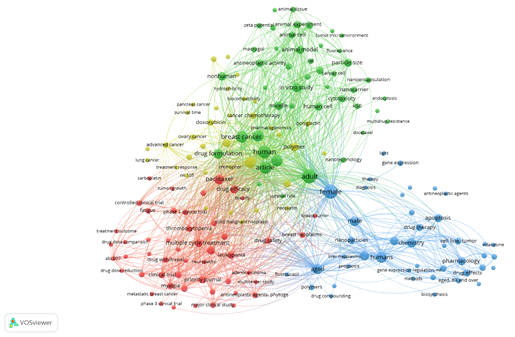
Figure 3 Visualization of the keyword network constructed by fractional counting from the 25 scientific works found in the Embase, PubMed/MEDLINE and Scopus databases, from 2011 to 2021, with their respective search strings. Note: its recurrences and connectivity between specific terms (analysis in VOSviewer 1.6.8 (2018) software).
Risk of bias and quality of evidence
The assessment of the quality and risk of bias of the studies discussed in this systematic review was conducted according to the method presented in each study, in which randomized clinical trials were evaluated and defined as: low risk of bias, not informed, some concerns or high risk of bias, using the RoB 2.0 tool, that through the algorithm judged the risk of bias of the articles Ranade et al.20 and Fujiwara et al.,21 based on the questions in each domain. Therefore, we observed that the two randomized clinical studies were classified as low risk of bias and points to a high quality of studies for all 5 domains (Figure 4a), in addition, in Figure 4b we see that the articles are classified at 100% as low risk of bias, increasing the reliability of the results presented in this review.
For non-randomized clinical trials, we observed that domain 2 for the study by Gaur et al.22 was classified as “Moderate” and in the study by Shi M et al.23in domain 6 as “Serious”, as illustrated in Figure 5a. However, we observed that it did not compromise the quality of the studies in the overall assessment and that the risk of bias was considered low for both articles. However, we highlight that there is a 50% chance that there is a serious risk of bias for domain 6 “Bias in measurement of outcomes”, as seen in Figure 5b.
In the evaluation of preclinical studies, the “GENERIC” tool of the Robvis software18 showed that the study by Fulfager & Yadav24 has a high risk of bias (Figure 6), which makes perfect sense, as the authors investigated the co-delivery of therapeutic agents in a nanocarrier to combat multidrug resistance (MDR) in breast cancer through an integrative review study. Thus, the software pointed to domains 2 and 5 “not clear” and to domain 4 “serious risk of bias”, as we can see in Figure 6a.
In addition, we observed that all preclinical studies discussed in this systematic review are of high quality for domains 1, 3 and 7, presenting “low risk of bias” in up to 100% of their results, as shown in Figure 6b. We also highlight that there is a chance of 45% of the studies not presenting clarity regarding domain 2 and a rate of 10% for domains 4, 5 and 6, respectively. In addition, there is a 10% “serious risk of bias” for domains 4 and 6, in addition to a lack of information of up to 55% for domain 2, as shown in Figure 6b.
Breast cancer has been the subject of several studies. Depending on its prognosis and stage of the disease, conventional therapies such as chemotherapy, radiotherapy and surgeries may be ineffective. In addition, they are invasive treatments orcanpresent high toxicity causing several side effects and/or adverse effects to the patient. Thus, the applicability of polymeric nanoformulations as carriers of antitumor drugs based on the literature of this systematic review, demonstrated therapeutic potential for the treatment of breast cancer, that is, a more effective, controlled and targeted delivery to the desired target was observed.
In studies developed by Abou-El-Naga et al.25 they performed in vitro assays using NPsP loaded with a drug (Docetaxel – DTX) that could be less toxic and more assertive in the treatment of breast cancer. For this, NPsP based on PLGA or poly(L-lactic acid-co-glycolic acid) was used precisely because of its biocompatibility and biodegradability characteristics. In conjunction with the drug Docetaxel, which is used in the antineoplastic treatment of various types of cancers, such as lung, prostate, head, neck, gastric, ovary, bladder and especially breast cancer. The tests showed that the drug DTX was effectively encapsulated in NPsP. The MTT experiments lasted twoweeks and showed that drug uptake depended on the time cells were exposed to it, reaching its maximum after activation of the endocytosis mechanism, which occurs when living cells absorb some material or substance through the cell membrane. It was also observed that cytotoxicity depends on the concentration used, and that the use of the PLGA-DTX system generates a greater apoptotic effect (which would be programmed cell death) through the activation of Caspase-9, Caspase-3 and P53 genes. Therefore, the proposed NPsP demonstrated a promising drug delivery system in the treatment of breast cancer.
In studies by Yap et al.,26 responsive photosensitive molecules called donor-acceptor Stenhouse adducts (DASA) were used to address disadvantages in the use of ultraviolet light, such as low tissue penetration. Wherein, the photoisomerization of DASAs results in changes in solubility, polarity and structure that draw attention to applications in various fields of chemistry. The toxicity of ellipticin-loaded micelles with and without light irradiation was tested using the MCF-7 human breast cancer cell line (in vitro). As a final result, the authors demonstrated that DASAs polymers are able to encapsulate ellipticin, but the amount of charge is limited, as it was observed that a high level of drug charge destabilizes the serum micelle and that drug-laden micelles are more toxic after 2 hours of irradiation compared to the non-irradiated sample. In addition, it was noticed that after 72hours of irradiation there was an increase in toxicity, indicating that the drug is being released continuously. Finally, drug translocation into breast cancer cells (MCF-7) was successfully performed by micelles.
In studies by Oda et al.,27 it was also observedthe effectiveness of the strategy of using NPsP for delivery of therapeutic potentials in target cells, in which researchers developed PM from DTPA (a lyophilized kit containing diethylenetriaminepentacetic acid (DTPA)-functionalized 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy (polyethylene glycol)-2000] containing paclitaxel (PTX) (MP-DTPA/PTX) to be used as a theranostic tool in breast cancer. The results of this study showed that the lyophilized kit was able to protect and maintain the physicochemical and radiochemical pharmacological properties of PM-DTPA/PTX until delivery to 4T1 murine mammary carcinoma cells, effectiveness of freshly prepared PM was also observed maintaining their biological profiles until delivery to the cell- target.
Another example of the strategy of using NPsP for drug delivery in the fight against breast cancer is reported in studies by Khan et al.,28 who synthesized a carboplatin-loaded chitosan NPsP (CPLCsNPs). Carbplatin is a chemotherapy drug used to treat patients diagnosed with breast cancer, however, this drug has serious side effects that in many cases its administration is suspended as it is life-threatening to very debilitated patients.29 In this way, the use of NPsP for the delivery of this drug can effectively decrease these serious side effects, in addition to increasing its therapeutic efficacy. Khan and colleagues28 observed that CPLCsNPs have greater efficacy compared to free carboplatin, these results could be evidenced from MTT analyzes of the cytotoxic activity of CPLCsNPs and free carboplatin in human breast cancer cell lines (MCF-7).28 In addition, it was also notedthat drug delivery by NPsPs is performed slowly, compared to free drug, which means that continued exposure and release of pharmacological properties by CPLCsNPs makes it more efficient by maintaining a longer period of time in the tumor.
On the other hand, in studies by Chidambaram & Krishnasamy,30 who performed encapsulation of Curcumin-Piperin (Cur-Pi), Curcumin-Quercetin (Cur-Qu) and Curcumin-Silibinin (Cur-Si) in NPsP, we observed in their results of in vitro cytotoxicity by MTT assays that the nanoformulations presented IC50 lower than pure curcumin. However, NPsP encapsulated with Cur-Si showed significant cytotoxicity on MCF-7 cancer cells compared to the PTX positive control. In contrast, in vivo rat assays showed that the encapsulated NPsP of Cur-Pi, Cur-Qu and Cur-Si exhibited increased biological activity in several mouse mammary cancer cell lines compared to pure curcumin.30 This may be related to the effectiveness of NPsP in protecting the active ingredient until delivery.31 Thus, the encapsulated NPsP of Cur-Pi, Cur-Qu and Cur-Si showed spherical morphology with excellent uniformity, which increases their aqueous solubility by more than 50 times compared to pure curcumin. In addition to the nanoformulations presenting a rate of more than 95% of drug release in 45 minutes in the gastric fluid, which may have contributed to a better performance of the nanoformulations than pure curcumin.
In the toxicity results in vivo assays, was noticedthat the polymeric nanoformulations of Cur-Pi, Cur-Qu and Cur-Si did not induce significant changes in cells that remained within the normal histopathological limits in anatomopathological examinations of vital organs such as the liver, kidney, heart and brain. This shows the safety of nanoformulations for drug delivery without causing adverse effects, in addition to having chances of improving drug efficacy, as observed in the other studies available in Table 2.
Manjili et al.32 performed an in vitro and in vivo pharmacokinetic investigation of Artemisinin-loaded PM (PCL-PEG-PECL-ART). PM formed by the Polyethylene Glycol (PEG) polymer was used, which has a wide range of applications in controlled drug delivery systems, together with an additive for polymers, Polycaprolactone (PCL), which is a cyclic, biodegradable, and low melting point, facilitating its experimental use. Because of its molecular structure, it manages to increase the solubility of Artemisinin (ART) in water, increasing its bioavailability. Thus, forming the PCL-PEG-PCL system, where the drug ART was encapsulated with an efficiency close to 90%. ART is a fast-acting drug used in anti-malarial and anti-tumor medications. In vitro and in vivo experiments were carried out in mice for approximately 31days. The results in vitro, MTT showed that the miscellaneous tested were not toxic to the cell lines tested. In vivo tests showed that the proposed formulation increased and concentrated the drug in the tumor with prolonged action via intravenous administration, proving to be a promising treatment.
In the study carried out by Xiao et al.,33 the aim of the work was to reducethe serious side effects related to the chemotherapy treatment of triple-negative breast cancer. The triple-negative name of this type of cancer is related to the fact that cancer cells do not have the most common hormone receptors (estrogen and progesterone), in addition to not producing the HER2 protein, which is commonly present in most cases. For this, a hormone releaser (LHRH) directed to the tumor receptor was used together with NPsP based on Cholic Acid and PEG (Polyethylene glycol, which is a polymer widely used in controlled drug delivery systems). Thus, becoming a microenvironment-responsive system for selective drug delivery, PTX was used. The proposed system demonstrates high efficiency in drug release, adequate size of NPsP, in addition to the colloidal stability required for release. In vitro experiments were performed with the cell line MDA-MB-231 and MDA-MB-435, and ex vivo and in vivo experiments in mice. In which, through the tests, it was evidenced that the NPsP did not present hematological, hepatic and renal toxicity (ex vivo). Fewer side effects, loading efficacy, distributed release and antitumor response were also found (in vivo). Showing that in comparison with other drugs normally used or used only the PTX-NPs system, without hormonal targeting (LHRH), the same benefits are not achieved.
In more complex studies involving human physiology, Gaur et al.,22 carried out a pharmacodynamic study to verify the possibility and efficacy of using NPsP based on cyclodextrin and camptothecin (Camptothecin-CRLX101), which is a nanodrug administered in the treatment of breast, prostate and pancreas cancer. NPsP was designed to slowly concentrate and release the aforementioned drugs in tumors over a longer period of time. Preclinical in vitro experiments and human clinical trials were performed with patients over 18 years of age, in confirmed phase of advanced solid neoplasms or in metastasis phase, for 4 weeks. Tumor biopsy was performed, and blood samples were collected from patients before and after treatment with CRLX101, as well as immunohistochemistry and pharmacogenomics testing. It was observed that the expression of several proteins (Ki-67, CD31) that are quantified throughout cancer treatment decreased after the administration of the nanomedication (CRLX101). The quantification of these proteins is important, as they are inversely proportional to patient survival. Furthermore, CRLX101 demonstrated promising antitumor activity in pre-treatment, in solid or recurrent tumors, as it inhibits the proliferation of malignant cells. This effect is related to the slow release of the drug. However, the treatment showed a certain level of gastrointestinal toxicity, hemorrhage, and cystitis, but the authors point out that even with these side effects, it was not necessary to interrupt the treatment.
Another essential research in this discussion was conceived by Fujiwara et al.21 in which they carried out a randomized study, in which they compared the use of a new formulation of PM called (NK105) with the use of a drug already quite recurrent in the treatment of cancers, PTX, in patients with breast cancer metastatic or recurrent. The joint use of NK105-PTX was tested to decrease the level of toxicity and adverse reactions of PTX. The in vivo, randomized trial was carried out for approximately 28days to verify the efficacy and safety of using NK105 and PTX. A total of 436 female patients, aged 20 to 74years, were enrolled, who were confirmed in histopathological studies of metastatic breast adenocarcinoma. Patients were randomly assigned 1:1 to receive either NK105 (65 mg/m2) or PTX (80mg/m2) on days 1, 8, and 15 of a 28-day cycle, and observed their survival without disease progression. It was noted that the overall survival of patients averaged 31.2 to 36.2 months, the two groups exhibited similar safety profiles, but NK105 performed better in terms of toxicity than PTX as reactions were observed such as grade 3 infections, febrile neutropenia, and severe thrombocytopenia.
Still on PM, in the article by Shi et al.23, was developed a pharmacokinetic study of the formulation of PM associated with PTX medication (PM-PTX) for intravenous injection in patients with advanced stage solid malignancies. The main objectives of the work were to determine, through the test performed, the limiting toxicity, and the maximum tolerated dose of the drug. It was also possible to evaluate the safety of the treatment, and the antitumor activity in patients with advanced stage disease. Clinical trials were performed using two different concentrations of PM-PTX, a total of 18 patients participated in the study, 9 men and 9 women. Of these, 17patients had lung cancer, and one of them had breast cancer. There were 3weeks of drug administration, and 11months of observation of adverse effects. The first concentration administered was 175 to 435mg/m2 for 3hours without premedication on day 1 of a 21-day cycle. In a phase I clinical trial, it was demonstrated that PM-paclitaxelfor injection, a new taxane free nonionic emulsifier polyoxyl 35 castor oil (PCO) formulation, showed high maximum tolerated dose (MTD) without additional toxicity and exhibited preliminary antitumor activity. The main dose-limiting toxicities (DLTs) were neutropenia and peripheral sensorineural numbness. The results of the dose escalation trial and pharmacokinetic studies showed that PM-paclitaxel for injection is superior to conventional paclitaxel. At the end of this phase I study, the authors observed that the treatment had a desired antitumor activity in reducing tumor size, but with hematologic and non-hematologic adverse effects, such as anemia and transient skin reactions.
Thus, a new phase II clinical trial for PM-paclitaxel combined with cisplatin versus SB-paclitaxel plus cisplatin as a first-line treatment in patients with Advanced Stage Non-Small Cell Lung Carcinoma (NSCLC) is ongoing with a standard dose administration of 300 mg/m2 with intravenous infusion over 3hours every 3weeks to elucidate new results.
In the work developed by Ranade et al.,20 a multicenter randomized phase II study of a NPsP formulation formulated in the polyethoxylated nonionic surfactant castor oil with PTX was performed in women with locally advanced and/or metastatic breast cancer after anthracycline failure, PTX-containing NPsP in different concentrations proved to be safer and more effective in these patients. Thus, it was possible to conclude that NPsP safely allows for 1hour longer infusion of higher doses (220mg/m2) of PTX without premedication compared to standard PTX therapy (175mg/m2) with a 3-hour infusion schedule. Another important result observed was the NPsP with a concentration of 220mg/m2, whose neutropenia rate was lower when compared to cremophore and PTX. Finally, the incidence of grade 3 neuropathy was lower in NPsP at 220mg/m2 than in free PTX. The study demonstrates that NPsP at a dose of 220mg/m2 is comparable in efficacy and has a better safety profile than chromophore and PTX (175mg/m2).
Ultimately, we highlight a review study carried out by Fulfager & Yadav,25 who discussed several NP-based researches, including NPsP, with the aim of understanding how the co-delivery of therapeutic agents in nanocarriers works to combat multidrug resistance in breast cancer. In the research, the authors concluded that the co-delivery of different therapeutic agents can help in the modulation of intracellular ABC transporter proteins and in the silencing of multidrug resistance genes, thus affecting apoptosis, efflux and reducing the toxicity of chemotherapy. The NPsP have more attractive characteristics for use, since they have more adjustable physicochemical properties, have greater stability, have a more controlled drug release and sustaining profile, have a more homogeneous size and greater carrying capacity of a drug that it is poorly soluble in water. Finally, the authors state that this co-delivery of drugs with NPs has many benefits, but it is necessary to carry out extensive biological evaluations and understand the molecular mechanisms in detail.
The systematic argument of the bibliographic searches of this study was obtained from the analysis of 12 studies that bring together pre-clinical results in vitro, ex-vivo and in vivo (mice and rats) and clinical trials (patients with confirmed diagnosis of breast cancer and/or relapsed patients), which indicate the effectiveness of the use of polymeric nanoformulations for the delivery of drugs to the target site in the treatment of breast cancer. In this strategy improved the performance of the drug, having a greater concentration of the active ingredient in the tissues cancer patients, in addition to the increase in drug uptake and cellular concentration, as well as in sustaining their intracellular retention, which increases the effe
In this sense, it was also observed that NPsP decreased the side effects, considering that the active ingredient of the drug remains longer in the cancerous tissue, so smaller amounts of antitumor drugs are administered to the patient, offering a concentrated delivery in the affected area long-term. However, additional clinical studies are essential to confirm the use of polymeric nanoformulations in drug delivery in the treatment of breast cancer.
The authors thank the Universidade de Brasília - UnB for encouraging research and extension. We also thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - CAPES for the scholarship and the Research Group in Química Medicinal e Biotecnologia - QUIMEBIO of the Universidade Federal do Maranhão - UFMA, University Campus of São Bernardo, MA.
This study did not involve the participation of humans or animals and therefore did not need to be approved by an ethics committee.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
The authors declare no conflicts of interest regarding the publication of this paper.
Araújo, Joabe Lima, 2022, "Replication Data for: Advantages of the use of polymeric nanoparticles in the treatment of breast cancer: a systematic review", https://doi.org/10.7910/DVN/4MIQXC, Harvard Dataverse, V1.

©2022 Vieira, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.