International Journal of
eISSN: 2381-1803


Research Article Volume 17 Issue 1
1Coordinator of the ICHP Outpatient Clinic, São Paulo Municipal Public Servant Hospital (MPSH-SP), Brazil
2Professor at Alkhemy Lab by Joel Aleixo, and Volunteer Collaborator at MPSH-SP, Brazil
3Coordinator of the Executive Center for Planning and Quality at MPSH-SP, Brazil
4Teaching and Research Manager at MPSH-SP, Brazil
5National Reference Center for Traditional, Complementary and Integrative Medicines, Brazil
Correspondence: Joseli Beatriz Suzin, Av dos Eucaliptos,217, ap.62, Indianópolis, São Paulo, SP, Brazil
Received: February 02, 2024 | Published: February 19, 2024
Citation: Suzin JB, Santomauro AC, Campos FJB, et al. Use of a natural compound based on essential oils as a potential complementary therapeutic approach to smoking cessation. Int J Complement Alt Med. 2024;17(1):26-31. DOI: 10.15406/ijcam.2024.17.00679
Smoking is a major public health concern, given that the global number of smokers continues to rise, causing almost 8 million deaths worldwide in 2019, with one in five deaths being male. Therapeutic interventions that consider integrative health approaches have shown promising results in helping people to stop smoking, such as aromatherapy. Considering the potential of aromatherapy in interventions aimed at contributing to smoking cessation, which have already been described in the literature, the aim of this study was to evaluate a natural compound based on essential oils as a complementary therapeutic approach to help stop smoking. The design of this study is characterized as observational and was developed using a qualitative-quantitative approach with the participation of 15 individuals who had made the decision to quit smoking. This pilot study was coordinated by the Integrative and Complementary Practices outpatient clinic at the São Paulo Municipal Public Servant Hospital (MPSH-SP), a large, tertiary-level public care hospital in the city of São Paulo, Brazil. In summary, it was observed that anxiety, discouragement and stress were the symptoms most reported by the participants and were observed in 90% and 60% of those interviewed, respectively. Diseases such as diabetes, hypertension, respiratory diseases and other conditions were also present among the participants. Analysis of the Fagerström test showed that there was a reduction in the score classifying the degree of nicotine dependence, indicating a possible improvement after using the natural product under study. Despite the small sample size, this study points to promising evidence regarding the use of aromatherapy as a complementary approach to help stop smoking.
Keywords: Traditional Complementary and Integrative Medicines, Integrative and Complementary Health Practices, Chronic diseases and non-communicable diseases, Primary Health Care, Aromatherapy, Smoking cessation, Traditional and complementary medicine interventions
NHSA, national health surveillance agency; REC, research ethics committee; MPSH-SP, são paulo municipal public servant hospital; MoH, ministry of health; TCIM, traditional, complementary and integrative medicines; SWHC/MPSH-SP, specialised workers' health centre/são paulo municipal public servant hospital; SDG, sustainable development goals; WHO, world health organization; PAHO/WHO, pan american health organization/ world health organization; SAP-CDNCD, strategic action plan for tackling chronic diseases and non-communicable diseases in brazil; SCO/MPSH-SP - specialized center for occupational health/ são paulo municipal public servant hospital; ICHP, integrative and complementary health practices; NPICP, national policy for integrative and complementary practices; FICF, free and informed consent form
According to the World Health Organization (WHO), tobacco can lead to the death of up to half of its users who do not quit smoking, with an estimate of more than 8 million deaths each year, including around 1.3 million non-smokers who are exposed to secondhand smoke. WHO estimates indicate that around 80% of the world’s 1.3 billion tobacco users live in low- and middle-income countries. In 2020, approximately 22.3% of the world’s population used tobacco: 36.7% of men and 7.8% of women.1 The WHO report on the global tobacco epidemic states that the global prevalence of smoking reduced from 22.8% in 2007 to 17% in 2021.2
According to data published in The Lancet Public Health in 2021, 82.6% of current smokers started smoking between 14 and 25 years old, with 18.5% smoking regularly before 15 years old. The study also points out that, in the absence of intervention, the annual number of 7 to 69 million deaths and 200 million disability-adjusted life years attributable to smoking will increase in the coming decades.3
Most studies on smoking show that nicotine is the main agent responsible for tobacco dependence. By binding to cholinergic receptors present in the autonomic ganglia, neuromuscular junction and central nervous system, prolonged exposure to the substance generates molecular changes and behavioral sensitization, which creates dependence.4
Eliminating such a habit is always a major challenge for all the methods that aim to help smokers in this endeavor. In this context, aromatherapy use can be a strategy for smokers who are in a smoking cessation program for helping to eliminate withdrawal symptoms such as anxiety, irritability, aggression, sleep disturbances, difficulty concentrating, sadness, depression or even eliminating cravings (a strong desire to smoke). And it is precisely this withdrawal syndrome that makes it most difficult to quit smoking.
Following WHO recommendations and observing the United Nations Sustainable Development Goals (SDGs), in particular SDG 3,5 the Ministry of Health (MoH) defined reducing the prevalence of smoking by 40% as a goal in its Strategic Action Plan for Tackling Chronic Diseases and Non-communicable Diseases in Brazil - 2021/20230 (SAP-CDNCD).6
SAP-CDNCD strategic actions interact with different laws, policies and guidelines published by the MoH, of which the Brazilian National Policy on Integrative and Complementary Practices (PNPIC - Política Nacional de Práticas Integrativas e Complementares) stood out. This policy, in turn, recommends guidelines for implementing integrative and complementary health services nationally. Some of these practices are part of the scope of what the WHO calls Traditional, Complementary and Integrative Medicines (TCIM). Currently, the MoH recommends 29 ICHP as complementary resources in healthcare, of which aromatherapy is included.7
The aromatherapy instrument comprises volatile concentrates, known as essential oils, which are organic plant compounds formed by highly complex molecules that have various chemical functions, the prevalence of which determines their aroma. These substances are used to bring physical, mental and emotional well-being.8
Considering the potential of aromatherapy in interventions that aim to contribute to smoking cessation already reported in the literature, whether as a single option or combined with other interventions, the present work aimed to assess a natural compound based on essential oils as a complementary therapeutic approach to help in smoke cessation.
Study site
The present study was carried out at the ICHP outpatient clinic at Municipal Public Servant Hospital in São Paulo, Brazil, in partnership with the Specialized Center for Occupational Health (SCO/ MPSH-SP).
It is a large, tertiary care public hospital in the city of São Paulo, located at Rua Castro Alves, nº 69, in São Paulo, SP, Brazil, since 1957. It is a public, autonomous entity, linked to the Municipal Health Department of this municipality. MPSH-SP service is exclusive to active and inactive public servants and municipal public employees, their dependents and pensioners of the Direct Administration, Municipal Authorities, Municipal Chamber and Municipal Audit Court. Its purpose is to provide medical, dental and pharmaceutical care to municipal public servants, providing inpatient and emergency clinical-outpatient care. It is also an institution that offers training to healthcare professionals as well as provides 24-hour emergency medical care to the general population in adult and pediatric emergency rooms.9
The hospital’s physical structure is composed of:
The ICHP outpatient clinic began with the opening of a meditation room in 1999, the first in a public hospital in Brazil. At that time, the ICHP outpatient staff offered the body practices of Traditional Chinese Medicine, with meditation as the main activity. Over time, the most varied therapies were incorporated, such as body practices such as Tai Chi Chuan, Lian Gong, “laying on hands” techniques such as Reiki, access bar, neural stimulation, floral therapies, systemic constellation and many others. We currently offer access bars, systemic constellation, attitudinal healing, neural stimulation, phytotherapy, alchemical flowers, hypnotherapy, anthroposophic medicine practices, meditation, Reiki, self-care therapy, thought field therapy.10
All work is carried out through a volunteer program, where professionals specializing in these practices register and carry out the work according to the organization and need identified by the coordination of the ICHP outpatient clinic. In the space’s 25 years of existence, more than 150,000 services have been provided.
This work led to recognition from the Rotary Club of São Paulo, granting an award in 2021 to the coordinator for her notable services to the well-being of people’s lives by starting the 1st meditation room in a public hospital and later for the ICHP program.
The following year, in 2022, we received a national recognition award from MoH in partnership with the Pan American Health Organization/WHO (PAHO/WHO) through the Health Innovation Laboratory in Integrative and Complementary Health Practices (LIS-PICS - Laboratório de Inovação em Saúde em Práticas Integrativas e Complementares em Saúde). In this initiative, we were recognized as one of the six successful experiences of implementing ICHP in the Brazilian Health System.
Study design
The present study can be characterized as an observational pilot study and was developed using a qualitative-quantitative approach. Its development consisted of 5 stages, which will have their methodological aspects described below:
Stage I Project submission to the MPSH research ethics committee: This research followed scientific principles and ethical aspects involving human beings in accordance with the Brazilian National Health Council Resolutions 466/2012 and 510/2016 nationally.11
The study was registered on Platform Brazil on 08/27/2020 under number 37040020300005442 and was approved by the MPSH Research Ethics Committee (REC) on September 15, 2020, the date on which the project began. At this stage, it was decided that all individuals who agreed to participate in the study should be informed about the nature of the research and sign the Informed Consent Form (ICF whose models were previously approved by the MPSH REC. After providing the necessary clarifications, participants were invited to answer a questionnaire and undergo an interview. Both procedures were carried out by a qualified SCO/ MPSH-SP professional duly trained for these activities and/or by the researchers in charge of the study. All personal information collected was preserved and used only for the purposes of the study.
Stage II: Participant screening and questionnaire application: Participant screening was carried out in September 2020. The 15 selected participants were employees from various sectors of MPSH-SP that are served by SCO/MPSH-SP.
As eligibility criteria, they were established as mandatory:
The questionnaire was designed for individual application and included questions that basically addressed the following topics: a) personal identification; b) antecedents related to the health situation; and c) Fagerström test. The questionnaire used was customized from one already existing at MPSH-SP in an internal program called “Tobacco Free”, including the Fagerström test. This test, consisting of six questions, made it possible to assess the degree of nicotine dependence. Their questions are related to smoking cessation behaviors and have been considered useful in the discussion of nicotine dependence. It is a test that has already been validated and applied in different contexts, such as nicotine dependence among adolescents or university students, and was widely used in other contexts.12
Stage III: Delivery of the product to be used to participants and instructions for use:
Product characterization: The product used is commercially known as a spray by the name “immune synergy”, manufactured by AlkhemyLab by Joel Aleixo. Its formulation is composed of water, Mentha spicata extract, Lavandula angustifolia extract, Ocimum basilicum extract, Rosmarinus officinalis extract and essential oil, Eucalyptus globulus essential oil, Eugenia caryophyllus essential oil, Lavandula officinalis flower essential oil, Melaleuca alternifolia essential oil, Mentha piperita essential oil, Ocimum basilicum essential oil, linalool* and eugenol* (natural components of essential oils). The product does not contain ingredients of animal origin. At the time of our research, in September 2020, the product batch in use was number 0520311, with an expiration date scheduled for May 7, 2023.
Instructions for use: All participants were instructed by the doctors in charge of the study regarding the use of the product as follows:
Stage IV: Fagerström test repetition: At the end of six months after using the product, all participants were invited to return to SCO/MPSH-SP for an interview with the psychologist responsible for this activity to repeat the Fagerström test.
Stage V: Data assessment and statistical analysis: Information was collected using a printed form, and data were entered into an Excel spreadsheet. Statistical analysis was performed using the tools available in Excel 2007/Microsoft.
Of the total of 15 participants, it can be observed that 14 of them were female and one was male, with ages ranging from 28 years old (the youngest participant) to 62 years old (the oldest participant). In relation to marital status, it was observed that 6/15 (40%) declared themselves married, 4/15 (27%) were divorced/separated, whereas another 4/15 (27%) reported being single. Only one participant reported being a widow. As for education, 7/15 (47%) reported having completed high school, 6/15 (40%) reported having completed higher education and 2/15 (13%) did not complete higher education (Table 1).
|
Participant ID |
Age |
Civil status |
Education |
Occupation |
|
ID-1 |
48 |
married |
High school |
Public policy management assistant |
|
ID-2 |
53 |
divorced |
University degree |
Coordinator |
|
ID-3 |
47 |
divorced |
High school |
Public policy management assistant |
|
ID-4 |
58 |
married |
High school |
Public policy management assistant |
|
ID-5 |
54 |
married |
High school |
Electrician |
|
ID-6 |
47 |
married |
High school |
Public policy management assistant |
|
ID-7 |
46 |
married |
High school |
Public policy management assistant |
|
ID-8 |
54 |
widow |
University degree |
Public policy management assistant |
|
ID-9 |
41 |
married |
University degree |
Biologist |
|
ID-10 |
44 |
single |
University degree |
Public policy management assistant |
|
ID-11 |
29 |
single |
University degree |
Public policy management assistant |
|
ID-12 |
28 |
single |
Incomplete University degree |
Nursing assistant |
|
ID-13 |
58 |
single |
High school |
Nursing assistant |
|
ID-14 |
59 |
divorced |
Incomplete University degree |
Public policy management assistant |
|
ID-15 |
62 |
divorced |
University degree |
Nursing assistant |
Table 1 General profile of survey participants
Source: Prepared by the authors (2024).
Concerning family composition, it is observed that participants live in their homes with a number ranging from one to six people living in the same residence and that there is at least one smoker in each house. Of the 15 participants, only three did not answer this question. Data showed that the habit of smoking has been present in participants’ lives since adolescence, with all participants having smoked for at least 20 years.
When asked about the motivation that led them to start smoking, all participants reported that they had their first contact with cigarettes due to the influence of people close to them, such as friends, relatives or co-workers. Only one participant (1/15=7%) reported that they had never taken any initiative for smoking cessation, whereas all other participants (14/15=93%) reported having already taken any initiative for smoking cessation, either with help from medications, patches or other unconventional approaches such as acupuncture and laser. Since the initial attempt to quit smoking was unsuccessful, 14 participants (14/15=93%) reported that they returned to the habit for various reasons, all related to emotional factors such as stress, anxiety and worry. Due to pregnancy, one participant (1/15=7%) reported having quit smoking, however, as soon as the gestational period ended, she resumed smoking.
Different motivations for participating in this research were reported, with the exception of one participant who did not answer this question (1/15=7%). Among the motivations reported, those related to the need to quit smoking were the most frequent (9/15=59%), followed by reports of health problems (4/15=27%), in addition to one case in particular who reported being repulsed by the smell that cigarettes leave on people who smoke (1/15=7%).
It was observed that the vast majority of participants (14/15=93%) reported smoking more frequently at home than at work (1/15=7%). Distinct situations that drive participants’ smoking habit were reported, such as situations of stress, anxiety, worry, sadness, depression, after meals (especially lunch), after drinking coffee, when experiencing moments of idleness.
Concerning the symptoms reported by participants, there were at least two of the following symptoms described by participants, among which anxiety was the most frequent symptom (14/15 = 93%), followed by discouragement (9/15= 60%), stress (9/15=60%), irritation (8/15=53%), sadness (8/15=53%), insomnia (7/15=47%), reduced concentration (7/15=47%), feeling of emptiness (6/15=40%), crying easily (3/15=20%) and aggressiveness (1/15=7%) (Figure 1).
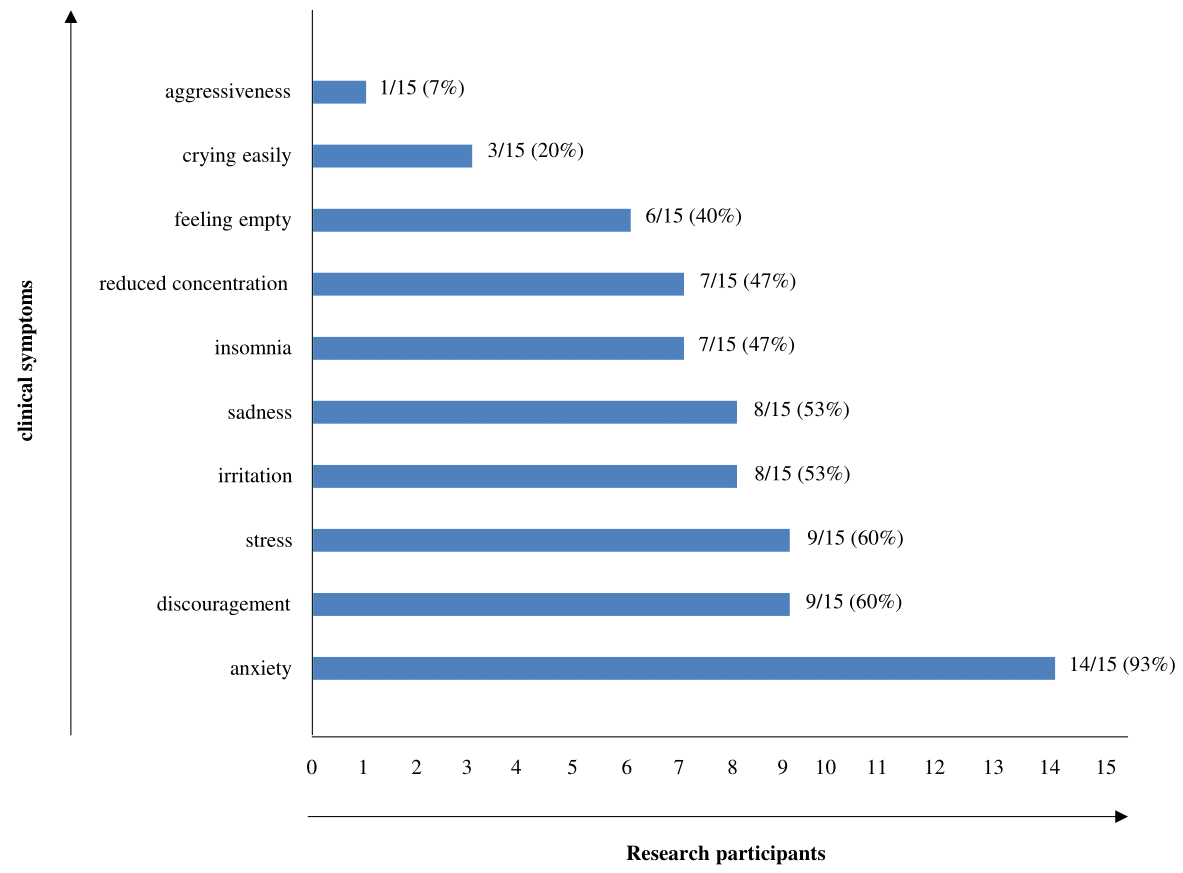
Figure 1 Symptoms self-reported by participants during the interview held at the MPSH-SP in São Paulo/Brazil, during September 2020.
Source: Prepared by the authors (2024).
In clinical history assessment, it was possible to identify the following profile of participants: 4/15 (27%) reported having allergies to medications such as acetylsalicylic acid, amoxicillin and also to other components, such as pollen and dust. Meanwhile, the other 11/15 participants (73%) claimed not to have any type of allergy. When the prevalent diseases and/or diseases were investigated, the presence of diabetes, hypertension, respiratory and vascular diseases as well as altered cholesterol and triglyceride levels was identified. No cases of neoplasms or heart disease were reported.
On the other hand, 10/15 participants (66%) reported having already undergone surgery for various causes, such as gallbladder, appendix, rhinoplasty, spine, cesarean section, hemorrhoid, rhinoplasty, bariatric, knee and hysterectomy, ovarian surgery, diaphragm hernia, esophageal hernia, hysterectomy and kidney stones. As for specific medication use, 7/15 participants (47%) reported using medications prescribed by doctors for diseases/conditions reported above.
The first FT application demonstrated that most participants (8/15=54%) reported smoking their first cigarette within the first five minutes after waking up. The other part of participants reported smoking between 6 and 30 minutes after waking up (3/15=20%), between 31 and 60 (2/15=13%) minutes after waking up or only after 60 minutes after waking up (2/15=13%). In relation to smoking in prohibited places, 6/15 (40%) participants mentioned that it was difficult to smoke in prohibited places, while 8/15 (60%) reported the opposite.
When asked which cigarette of the day provides the greatest satisfaction, 11/15 (73%) participants reported that it was the first cigarette smoked in the morning that provided the greatest satisfaction. The remaining participants (4/15 = 27%) reported having greater satisfaction when smoking other cigarettes throughout the day. Regarding the most frequent period of smoking, 5/15 (33%) participants reported that they smoke in the morning, whereas 10/15 (67%) reported smoking at other times.
Regarding maintaining the habit of smoking even in critical health situations, 11/15 (73%) participants mentioned smoking even when they are sick or bedridden, whereas another 4/15 (27%) said they do not smoke in these critical situations.
When assessing the level of physical dependence on nicotine as well as the tolerance and compulsion process using the Fagerström test (FT), we observed that all 15 participants responded fully to the FT in its first application. However, at the end of this study, in which this test was applied for the 2nd time, we identified withdrawal of seven participants for different reasons, such as: a) non-adaptation to the product (1 participant); b) intolerance to Melaleuca alternifolia (1 participant); c) headache and sinusitis (1 participant); pregnancy (1 participant); and d) three participants did not respond to the call to repeat FT, being considered as “dropouts”. With these seven cases of withdrawal, it was not possible to comparatively assess FT application at the two moments proposed by the study: at the beginning, prior to using the target product of the investigation and, at the end, after using said product (Table 2).
|
Repetition of Fagerström test |
no |
% |
||
|
Participants who responded |
8 |
54% |
||
|
Participants who reported reasons for giving up |
4 |
26% |
||
|
Participants who did not answer |
3 |
20% |
||
|
Total |
15 |
100% |
||
Table 2 Sample characterisation regarding the second application of the Fagerström test at the end of the study
Source: Prepared by the authors (2024).
It was possible to comparatively analyze the FT of the eight participants who remained in the study until the end, and the results indicate an interesting scenario, in which it is possible to notice that there was a reduction in the score, indicating a possible improvement with use of the immune synergy product (Table 3).
|
Participant ID |
Score |
Addiction classification |
Score |
Addiction classification |
Medium score |
Identification of dependency according to the average of the 2 tests applied |
|
|
FT1 |
FT |
FT2 |
FT |
FT1 + FT2 |
|
|
ID 2 |
1 point |
very low |
1 point |
very low |
1 point |
very low |
|
ID 4 |
0 point |
very low |
0 point |
very low |
0 point |
very low |
|
ID 5 |
7 points |
high |
7 points |
high |
7 points |
elevada |
|
ID 7 |
4 points |
low |
1 point |
very low |
2,5 points |
low |
|
ID 8 |
3 points |
low |
2 points |
very low |
2,5 points |
low |
|
ID 10 |
6 points |
high |
4 points |
low |
5 points |
low |
|
ID 14 |
7 points |
high |
4 points |
low |
5.5 points |
low |
|
ID 15 |
7 points |
high |
5 points |
medium |
6 points |
medium |
Table 3 Comparative evaluation of the Fagerström Test (FT) before and after the use of the Immunosynergy (Alkhemy Lab) product in eight participants
Source: Prepared by the authors (2024).
ICHP can be considered as a broad set of healthcare practices based on theories and experiences from different cultures used for health promotion, prevention and recovery, taking into account the whole being in all its dimensions.16 WHO recognizes their potential as therapeutic resources for health recovery, being recommended as a complementary approach to be implemented in Primary Health Care Services of the health systems of their respective Member States.2,17
Different studies point to the promising effectiveness of using interventions based on TMIC as a complementary strategy to help quit smoking. In this context, aromatherapy has been identified as one of the approaches with a simple application methodology, with promising results, and its benefits deserve to be further investigated.18–20
As demonstrated in the present study, the motivations that lead people to smoke are different, and can be driven by emotional issues, for the most part, but also by the influence of people close to them and curiosity. Rocha et al.21 identified a relationship between greater smoking and motivational profile, including the smoking pleasure, tension reduction and physical dependence domains, contributing to developing new strategies for smoking cessation.
It is known that the therapy proposed by the effects of essential oils is widely known for contributing to reducing stress and anxiety levels, and is therefore frequently recommended due to the relaxation provided by inhaling the active ingredients of these essential oils, derived from a complex mechanism of chemical molecules on the limbic system and hypothalamus releasing neurotransmitters.22 Although we identified two participants who withdrew because they reported “intolerance to some product component” or “sinusitis attack”, it is known that in general using essential oils is a safe therapy, with minimal side effects, as long as route of administration, correct dosage and chemical properties of the active ingredients of each oil are carefully observed. The extracts and essential oils of the plants that make up the product assessed in this research are already very well studied in the Brazilian Pharmacopoeia, providing the necessary safety to use these plants considered medicinal.23,24 Allergic reactions are rarely reported, but these oils are not free from oxidation reactions. Over time, changes in composition of chemicals are reported when stored for a long time. When discussing safety, it is worth noting that no studies have yet proven that the essential oils used are harmful.25
Many people begin to seek some type of treatment when they realize that there is a need to quit their smoking habit, as it is negatively impacting some aspect of their health. Despite the small sample size, this scenario was observed in more than 60% of the population studied in the present work and was also observed in other studies.26
Participant screening regarding their clinical history demonstrated a profile where diabetes, hypertension and respiratory diseases were identified, in addition to altered cholesterol and triglyceride levels. These results are indicators of attention when assessing smokers, given that such indicators can evolve into a degree of chronic morbidity that, in turn, can act as risk factors for serious health complications associated with complications of smoking. According to the Brazilian Diabetes Society, the main consequences of smoking for people’s health are chronic obstructive pulmonary disease and lung cancer, among other important cardiovascular complications, such as myocardial infarction.27 A systematic review study of 290 journals demonstrated the effectiveness of aromatherapy as a complementary approach to aid in anxiety and/or high blood pressure treatment.28
Internationally recognized, FT offers a quantitative measure, from 0 to 10 points, that assesses the degree of physical dependence on nicotine as well as the process of tolerance and compulsion: the higher the score obtained, the greater the degree of physical dependence on nicotine. The instrument can be used in the initial approach to users, acting as a trigger for reflections about their addiction process and the possibility of seeking treatment.7,13 Unfortunately, in the present study, it was not possible to compare the results of the two FT applications (at the beginning and end of the study), as only eight of the 15 participants were present to repeat this test, as previously reported. However, a possible comparative data analysis indicates that there was a positive effect after using the product, since there was a notable reduction in the degree of dependence in 63% of participants who responded to the repeat test, whereas the others (37%) showed no change in the degree of physical dependence on nicotine. These data, in principle, suggest that using the product may have had an effect on controlling the desire to smoke, which should be further investigated with larger study populations and using new methodology including case control. It was not possible to compare results with previous studies as there were no previously published studies, which makes this our pioneering study using the product in question.
Generally, studies with small populations do not allow us to generally infer the conclusions observed regarding the benefits observed. There is a consensus on the need to increase the number of participants to better ascertain the outcomes on all aspects observed. A questionnaire already validated internally by the HSPM clinical pulmonology staff was used, but it was not validated specifically for this research. When considering these limitations, the authors have already discussed the perspective of carrying out a clinical trial with a larger study population, previously validated questionnaires to expand understanding of the potential benefits observed with the immune synergy product use.
It is evident from the present study that the product used presents promising perspectives as a complementary and integrative approach to help smoking cessation, a prerogative that will be proven with a larger study population.
Aromatherapy is an important tool in the process of quitting smoking. The study showed that the natural compound product was effective in this objective, reducing nicotine dependence, reducing the number of cigarettes consumed per day, delaying the onset of cigarette consumption in the early hours of the day, increasing motivation to quit the habit by reducing withdrawal symptoms.
We would like to thank the partnership with the Specialised Workers' Health Centre in the person of the coordinator at the time the research began, doctor Eraldo J. R. Lima, and the psychologist responsible for the referrals and interviews, Mrs Valdecir F. C. Paes. We would also like to thank the partnership with the Alkemy Lab by Joel Aleixo for always being there when needed, providing information and donating the product for this study.
The authors Joseli Beatriz Suzin, Fernanda Julio Barbosa Campos and Juliane Cristina Burgatti are civil servants employed by the municipality in which the study was carried out. The other authors have no relationship to the research institution, Doctor Augusto Santomauro is a voluntary collaborator at the HSPM PICS outpatient clinic and Professor at the Joel Aleixo School of Alchemical Flowers, and Doctor Christiane Santos Matos has no employment or voluntary relationship to the institution where the research was carried out.
None.

©2024 Suzin, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.