International Journal of
eISSN: 2576-4454


Research Article Volume 6 Issue 5
1Large Biosphere-Atmosphere in Amazon Program, LBA/INPA, Brazil
2Department of Atmospheric Sciences, IAG/USP, Brazil
Correspondence: Rommel BC da Silva, Large BiosphereAtmosphere in Amazon Program, LBA/INPA, Brazil
Received: September 13, 2022 | Published: September 26, 2022
Citation: da Silva RBC, Gonçalves FLT. Statistical influence of climate on the population density of culex and coquillettidia mosquitoes. Int J Hydro. 2022;6(5):188-195. DOI: 10.15406/ijh.2022.06.00325
We carried out this study in the area of “Estação Científica Ferreira Penna” (FPSS), located in the Caxiuanã National Forest, in the state of Pará, eastern Amazon. This biome is considered one of the most important ecosystems with the greatest biodiversity of insects and plants on the planet. The climate records were obtained from the INMET meteorological database (BDMet), in addition to information from a microclimatic tower belonging to the Scientific Program “The Large Scale Biosphere-Atmosphere in Amazon Program (LBA)”. Mosquito specimens were sampled during the months of July, September and December 2005 and February and April 2006. During this period,»26,910 specimens were captured using the 'human attractant' method. A total of 59 species in 15 genera were captured and the genera Culex and Coquiilettidia were the focus of this study. The results show a statistical analysis of the impacts of environmental variables on the population density of mosquitoes of the Culex and Coquillettidia genera, emphasizing their eco-physiological behavior. There is a clear positive association between the concentration of atmospheric CO2 and the population density of these genera. When the rainy and dry seasons are analyzed separately, by the daytime period, the positive association is more evident.
Keywords: mosquitoes, temperature and relative humidity, amazon, climate change, CO2
The Amazon region is considered one of the most important and most diverse ecosystems on the planet. Brazil's two states, Amazonas and Pará, have the largest area in the Brazilian Amazon (Figure 1c). In addition to the equatorial forest, the region also has a small area of mangrove (on the coast) and some areas of transition to savannah, a type of ecosystem known locally as "cerrado". This region has 7% of the country's total population, with low population density,»2.91 inhabitants per km2. Most of the region's population (57.8%) lives in an urban environment.
Although with low human population density, the Amazon region has been largely deforested for the establishment of agriculture, livestock, logging, oil and gas prospecting, prospecting and mining. More recently, the forest has been heavily destroyed due to the construction of hydroelectric plants along the Amazon River basin.
The impact of human activities on the natural environment continually creates ecological conditions suitable for the expansion of mosquito habitats, as well as the pathogens that these mosquitoes can transmit.1 Worldwide, there are many examples of changes in the natural environment causing an increase in mosquito density, causing outbreaks of diseases associated with their pathogens, eg malaria and viral encephalitis.
It is widely accepted that changes are inevitable, however understanding the effects they will have on human resources and animal health can help in developing more appropriate strategies for land use and environmental management.1 As examples of the global impact of transmitted diseases, it is worth noting the 300 million cases of malaria per year, 50-100 million cases of dengue and 120 million cases of filariasis worldwide.2
The dynamics of vector-borne disease transmission is complex and involves an intrinsic system of interactions between insects, parasites and host animals that are associated with environmental determinants in contributing to the intensity of transmission.2 They addressed the impact of human changes on the natural landscape and the presence of An. Darlingi in some areas of the Peruvian Amazon.3 Observing that 41% of the wooded environments did not indicate the presence of An. Darlingi, while those environments, with the presence of these larvae, were sparsely forested with only 25% forest cover. Therefore, infection rates by An. Darlingi are higher in deforested areas compared to intact forest areas.4
The increase in the rate of stinging may be associated with the prevalence of these stings when infected by Plasmodium, causing changes in the feeding pattern of the insect.5 The deforestation process can also promote the emergence of previously unknown parasites and modify ecological conditions, causing changes in the relative abundance of vector species. Thus, it is possible to increase the transmission of infectious agents by species of mosquitoes favored by the changes that affect the behavior of the vector.
Deforestation can also encourage mosquitoes, which live in treetops, to disperse to ground level, increasing human exposure to infectious stings that often occur in the canopy. This may be the case of the yellow fever virus, with a risk of introducing this virus into urban areas.6,7
Among the emerging evidence of the impacts of climate change on human health is the modification in the distribution of some infectious diseases, including mosquitoes and their vectors, which cause changes in their seasonal distribution.8
The IPCC has projected that changes in air temperature, rainfall and other climate variables due to climate change are likely to affect the health of millions of people, particularly those with adaptive capacity, stating that there is great confidence that climate change currently contributes to global disease and premature death.9 Furthermore, changes in climate play a significant role in the distribution and prevalence of infectious diseases with important social impacts. This issue has increasingly attracted the attention of health professionals, particularly in the spread of malaria, among other vector-borne diseases.10
Evolutionary changes, where climate can affect the number of species, provide strong evidence for evolutionary responses related to reproduction with changes in insect “diapause” patterns, where there are new questions such as the conditions under which evolution can be expected, what Can factors constrain or promote evolutionary responses? When they occur, will they be enough to keep up with climate change?.11
The Amazon may experience warming and severe drought in the near future, where higher temperatures, as well as more sparse rainfall, directly affect all mosquito populations12,13 and.14
There are several genera of mosquitoes that are widely found in the Amazon region, including Culex and Coquillettidia, which are abundant and cover a large number of species. The genus Culex, described by Linnaeus in 1758, has the greatest variety of species in the Culicidae family, which can occupy a variety of niches15 and artificial habitats such as plant pots and plastic containers.16
Biologically, species of the genus Culex are poorly studied, consequently the epidemiological importance of this genus remains unknown. Some species are pests in urban areas, while others are involved in the dynamics of pathogen transmission to humans and other vertebrates.16 Among the Culex species of public health interest, it is important to mention those that participate in the transmission of parasites such as w. bancrofti that can cause filariasis;16 various arboviruses;17–20 and West Nile virus.21–23
The Coquillettidia genus is one of the genera that make up the Mansoniini tribe. The genus includes 57 species within three subgenera: Austromansonia (1 species), Coquillettidia (43 species) and Rhynchotaenia (13 species). Species of the subgenus Rhynchotaenia are restricted to the Neotropics.
Immature stages in the Coquillettidia life cycle rely on aquatic plants for oxygen. Females of several species are voracious and opportunistic,16 making them serious pests for humans and domestic animals. Some species are involved in the transmission of viruses to humans and domestic animals, Coquillettidia perturbans has been implicated as a transmitting vector of eastern equine encephalomyelitis virus in North America.24
Through simulations, the population dynamics of Culex tarsalis showed that the parameters, associated with the degree of dependence of temperature and density on larval mortality, were found to be an important component.25 When they related the density of Culex annulirostris and Aedes vigilax mosquitoes to environmental factors, they found an association with Ross River virus incidence in Brisbane, Australia.26
Checking the impact of atmospheric CO2 concentration on Aedes aegypti, the results show a climate sensitivity restricted by these concentrations, given that mosquitoes depend on carbon dioxide (CO2) to detect and orient themselves towards the blood of their host.27
However, variability and rapid fluctuations in atmospheric CO2 concentrations can affect the mosquito's host-seeking behavior. In this study, they analyzed the effect of transient levels of rising atmospheric CO2 on host search and on the behavior and physiological characteristics of the CO2-sensitive receptor olfactory neuron in female yellow fever mosquitoes (the Aedes aegypti).
The results of this study can help to predict future changes in mosquito-host interactions and, as a consequence, the vectorial capacity to climate change. Therefore, this study aims to characterize the impact of atmospheric variables, including the concentration of atmospheric CO2 on mosquito population density in primary forest (FLONA de Caxiuanã), in the state of Pará, eastern Amazon, with emphasis on the Culex genera. and Conquiletidia.
Climatic and geographic data
The climate of the studied region is classified as hot tropical climate, classified as 'Am' according to Köppen, according to Thornthwaite, type B'1 W'A, which is defined as a megathermal climate with moderate drought during the so-called "Amazonian summer", from July to November.
The study was conducted at the Ferreira Penna Scientific Station (FPSS), located in the National Forest (FLONA) of Caxiuanã, in the north-central part of the state of Pará, 400 km from the capital Belém. THEextension of the FLONA de Caxiuanã is 330 thousand hectaresof which 80% correspond to primary forests28 and low plateaus (terra firme); Várzeas, Igapós and, finally, the Natural Fields.29
The understanding of the hydrological cycle of the Amazon region increased due to the recent activities of the LBA scientific program, where important mechanisms in the formation of natural clouds and clouds influenced by particles from fires in the Amazon, were initially elucidated,30 observing extreme reduction in cloud formation with possible impact on the hydrological cycle.31
The FLONA area (6) is located between the cities of Melgaço (8) and Portel (7), in the north-central part of the state of Pará (Figure 1). Information on climate variability in this region was acquired from the meteorological database (BDMet) of the National Institute of Meteorology (INMET) from 1978 to 2010 in the municipalities of Altamira (1), Breves (2), Cametá (3 ), Porto de Móz (4) and Tucuruí (5), in addition to the state capital.

Figure 1 Location map of the Caxiuanã National Forest (6) and Ferreira Penna Scientific Station (blue circle), including the municipality of Melgaço (8), in the north-central part of the state of Pará, in the Amazon region.
Weather data
Meteorological measurements were obtained from a 52-meter high tower associated with the Large-Scale Biosphere-Atmosphere Scientific Program (LBA), whose coordinates are 1°43'9.9''S and 51°27'31.4''W , located in the FLONA area of Caxiuanã. The following environmental variables were recorded: concentrations of carbon dioxide (CO2 in ppm) and water vapor (H2O in ppmv), latent and sensible energy fluxes (LE/H in Wm-²), air temperature (Tar in °C), relative air humidity (in RH%), solar radiation balance (Rnet in Wm-²), atmospheric pressure (Pressure in hPa) and rainfall (PRP in mm), in addition to other variables not explored by this article.
These variables were collected in the same period in which the mosquitoes were captured. All climatic variables were sampled at half-hour intervals, however mosquitoes were captured at 1-hour intervals, which was the time chosen for this study. Insects were collected from July 2005 to April 2006, during 5 campaigns. Wet seasons (November to April) and dry seasons (May to October) as well as daylight data (7am to 6pm) and night time (7pm to 6am) were also analyzed.
To complement the data sets for the region, the variables air temperature, relative humidity and rainfall data were obtained from the database (BDMet) of the National Institute of Meteorology (INMET) in the municipalities of Altamira (1), Breves (2), Cametá (3), Porto de Móz (4) and Tucuruí (5) (Figure 1), at 00:00, 12:00 and 18:00 between the years 1978 to 2010.
Biological data
Mosquito specimens were captured in campaigns carried out during the months of July, September and December of 2005 and February and April of 2006. During this period,»26,910 specimens were captured using the “human attractant” methodology, with a total of 59 species distributed in 15 genera. Another 6,212 specimens were captured at night using light traps (CDC), this method collected 63 species in 8 genera. The genera Coquillettidia (1,212 specimens) and Culex (28,297 specimens) are among the largest numbers of individuals captured both by 'human attractant' and by light traps.
Principal component analysis (PCA)
Principal Component Analysis (PCA) is a way of identifying patterns in data and expressing them in a way that highlights their similarities and differences, assessed with correlation matrix analysis. The other main advantage of PCA is that once you have found these patterns in the data, you can reduce the number of dimensions without losing information. The goodness of fit depends on the number of components retained in the system and can be measured by evaluating the proportion of total variance captured by these components. There are several uses for PCA, the most important use is probably in multiple regression models, reducing the multicollinearity problem. In this study,.
Two PCA analyzes were performed in this study. In the first analysis, a dataset was applied to the raw material without the application of the rotational factor. In the second, hourly anomalies were applied, derived from the monthly average. Hourly data were subtracted from the average hour and these data were analyzed considering the application or not of the gross rotation (VARIMAX), when it allowed to clarify the general explanations.
Considering the number of independent variables (30), approximately half the number of days (out of a total of 360 hours analyzed), all factor loadings are close to and above 0.24 and can be considered significant for all PCA analyses. . The overall statistics results presents the overall correlation matrix, followed by principal component analysis (PCA) with raw and anomaly data, and finally the results of the time variability of the factor score.
The climate of the region
The air temperature
The equatorial climate characterizes the region with low variation and high temperatures throughout the year (average of 27°C to 29°C), which is typical for the northern region of Brazil. The air temperature patterns (figure 2) in the study area (Figure 1) include the municipalities of Altamira (1), Breves (2), Cametá (3), Porto de Móz (4) and Tucuruí (5), in addition to from the state capital, Belém Figure 2.
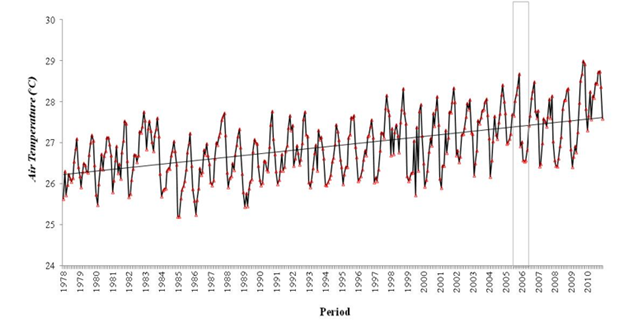
Figure 2 Average air temperature, the average of the 5 cities studied, plus the capital of the state of Pará in the period from 1978 to 2010. The rectangle corresponds to the sampled period. The visible rise in air temperature over decades in the region is due to the constant clearing of the forest with subsequent introduction of fire. The practice of fire is still used for the introduction of livestock in the Amazon.
The highest average annual temperatures are for the municipalities of Tucuruí 28.4°C, Porto de Móz, 27.9°C, Breves 27.8°C and Belém 27.5°C which occurred in 2010, followed by Altamira 28.2°C in 2005, and Cametá 28.1° C, in 1998. The lowest average temperature occurred in the municipality of Breves, 25.5°C, followed by Belém in 1982, 25.6°C and Porto de Móz 26.0°C, both in 1985. Altamira had the lowest air temperature in the period , 25.8°C in 1992 followed by Tucuruí, 26.2°C in 1999.
The second highest temperature in the 32-year period was recorded in 2005 in the municipality of Altamira, and the increase in air temperature this year can be partially explained by the phenomenon of severe drought that occurred in the western region of the Amazon,32 affecting the limit of the eastern region, showing that in 2005 there were anomalously warmer temperatures on the surface of the tropical Atlantic, a trend that had already been present since 2003, while the surface temperatures of the equatorial Pacific were almost normal.
All trends show a positive increase, showing that the region presented an average increase of»0.3°C, in the municipalities, where only the state capital (Belém) showed an average increase of»0.2°C per decade.
The relative humidity of the air
The annual variability of the relative humidity of the air with its respective linear trends (Figure 3) presents themunicipality of Cametá with the highest relative air humidity index, with an annual average of»88.3%, while the municipality of Altamira had the lowest (»85.1%). The relative humidity ofall municipalities show a clear downward trend, ranging from -0.07% to -0.46% per year, associated with a rising trend in air temperature, suggesting a real change in the climate. Cametá, Altamira and Tucuruí are part of the municipalities that are inserted in the line of the arc of deforestation.

Figure 3 The average Relative Humidity of the Air of the region in the period of 1978 to 2010, the average of the 5 cities of study, plus the capital of the state of Pará.
The rectangle corresponds to the sampled period of mosquito capture and the space corresponding to the years 1984 to 1986 indicates loss of information in this period. There is a reduction in atmospheric humidity levels due to the systematic increase in air temperature, suggesting that the climate in the region is tending to become drier, which will affect local biodiversity in the future.
The municipalities present their geographic and climatic characteristics according to the year of occurrence of adverse events, as they present a differentiated variability taking into account their location and proximity to water bodies. Porto de Móz recorded the highest average annual relative humidity in 1982, with 94.4%, followed by Tucuruí with 91.5% in 1986, Belém with 89.5% in 1988, Breves with 89.4%, Cametá with 88.3% in 1980 and Altamira with 85.1% in 1979. The lowest annual average amount of water in the atmosphere was recorded in the municipality of Altamira, with 75.2% in 2005, followed by Tucuruí with 76.4%, Cametá with 82.8%, Breves with 83.6% in 2010, Porto de Móz with 80.7% in 2009 and Belém with 82.3% in 2003.
According to the analysis, the trend lines show that the relative humidity of the air registered a global decrease, per decade, in this area of the Amazon region, being 1.1% in Altamira, 1.2% in Breves, 0.9% in Cametá, 2.6% in Porto de Móz, 2.4% in Tucuruí and 1% in Belém, although the percentages are low, there is a strong indication of a tendentially drier and warmer climate, especially in the second semester (dry period).
The rain
The annual variability of rainfall in the region is clearly separated by a dry season that runs from June to November, and another rainy season, covering the period from December to May. On the coast of the state of Amapá, near the mouth of the Amazon River,ORainfall rates can vary from 2000mm to more than 3000mm annually.28 The municipalities covered by this study have their climatic characteristics according to their landscape.
The annual amount and distribution of rainfall in the region has changed over the decades. During the study period (1978-2010) significant annual increases were observed chronologically in Tucuruí (3176.9mm) in 2001, Altamira (2922.1mm) in 1984, Cametá (3402.6mm) in 1994, Breves (2889.8mm) in 2000, Porto de Móz (2880.8mm) in 2005 and Belém (3663.8mm) in 2006, while the lowest levels of rainfall were recorded in 1978 in Porto de Móz (204.8mm) and Cametá (811.4mm), in 1981 in Altamira (663.3mm). ), in 1983 in Belém (2314.5mm), in 1984 in Breves (1145.4mm) and in 1992 in Tucuruí (1477.2mm).
The trends are positive for all municipalities with a significant increase ranging from 90mm to 280mm, per decade. Porto de Móz had the lowest records in the years 2006 and 2010, with evidence of a dry period during the interval (2005/2006) of mosquito collection. The other municipalities did not register low values in the same period. The records indicate that, in addition to the year 2010 being one of the least rainy years, there is a significant increase in rainfall (Figure 4).
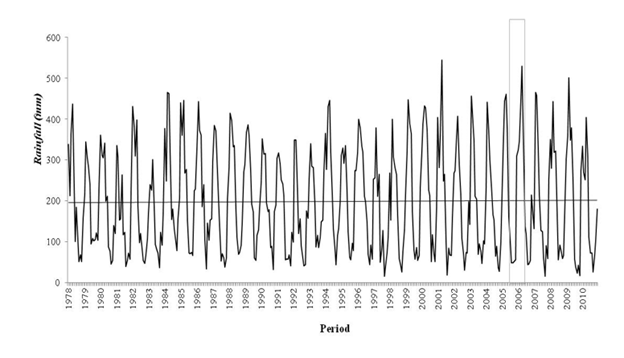
Figure 4 Monthly total rainfall from 1978 to 2010 in the region. The gray rectangle corresponds to the sampling period of mosquito capture. There is a marked increase in rainfall in the area, demonstrating an anomaly in the behavior of this variable that has been occurring for decades.
The analysis of rainfall behavior in the six municipalities indicated an average progressive increase in the region of ≈354.8mm.dec-1 per decade. The annual increase in the indices of air temperature and amount of rain with a consequent decrease in relative humidity suggests an indication that the climate in the Amazon is becoming hotter and drier, with a great possibility of an increase in the indices, intensity and frequency of rainfall affecting the hydrological cycle and the behavior of the region's biodiversity.
Temporal variability of culex and coquillettidia genera
The temporal variability of mosquitoes of the Culex genus (Figure 5a) shows a very high density compared to the Coquillettidia genus (Figure 5b), with the highest peak reaching 900 mosquitoes.h-¹ compared to 32 mosquitoes.h-¹ in the last specimens. The monthly variability is also different where, for the genus Culex, September had the highest density of individuals while July had the lowest. The genus Coquillettidia had the highest density of individuals in February and the lowest in December.

Figure 5 Temporal variability of Culex (a) and Coquillettidia (b) genera during mosquito collection campaigns. The “y” axis in red indicates the difference in scales between the vector species, showing the exaggerated abundance of one genus in relation to the other. The Culex genus was more abundant in the dry season (September) and the Coquillettidia genus was more abundant in the rainy season (February).
Correlation matrix
The correlation matrix indicates that both genders are significantly associated (Table 1). There is a positive but weak correlation between Culex and water vapor and a negative correlation with rain. Coquillettidia presents a negative correlation only with rainfall, as expected for mosquitoes in heavy rain situations, typical of equatorial areas.
|
Variable |
Correlations Marked correlations are significant at p < 0.05 N=320 (Casewise deletion of missing data) |
|||||
|
Culex |
Coquilettidia |
CO2 |
H2O |
tair |
rain |
|
|
Culex |
1.0 |
|||||
|
Coquilettidia |
0.53 |
1.00 |
||||
|
CO2 |
-0.07 |
0.02 |
1.00 |
|||
|
H2O |
0.15 |
-0.01 |
-0.24 |
1.00 |
||
|
tair |
0.02 |
-0.09 |
-0.32 |
0.08 |
1.00 |
|
|
rainfall |
-0.14 |
-0.21 |
-0.14 |
0.03 |
0.31 |
1.00 |
Table 1 Correlation matrix between mosquito genera and main variables
Statistical analysis between culex and principal component analysis (PCA)
The Culex genus has the highest mosquito population density during the period of severe drought in 2005, somehow responding positively to periods of reduced rainfall. The year 2005 was characterized as a year of severe drought in the Amazon, causing rainfall to be subjected to reduced rates in the period between one event and another.
Species of the Culex genus do not have resistance to the desiccation of their eggs.16 Consequently, the persistence of communities in periods of drought may depend on the acceleration of the developmental period of immature stages in an attempt to preserve their species by rapid population multiplication, i.e., the development time of immature stages may be reduced during a period of severe drought.
The factor associations and correlation coefficient between the variables and the modeled factors present the loads of unrotated components (Table 2) of the Culex genus associated with the data collected during the study period. The 1st factor, which explains 30% of the variance, has a non-significant positive association with gender (0.66) and water vapor (0.45) and a negative, significant association with air temperature (-0.71) and non-significant with air temperature. rain (-0.61). The 2nd factor, with 25% of the explained variance, presents a non-significant positive factor with Culex (0.41), significant with atmospheric CO2 concentration (0.86) and non-significant negative with water vapor (-0.56).
Unrotated |
Factor 1 |
Factor 2 |
R2 |
Culex |
0.66 |
0.41 |
0.13 |
CO2 |
-0.07 |
0.86 |
0.12 |
H2O |
0.45 |
-0.56 |
0.09 |
tair |
-0.71 |
-0.17 |
0.11 |
rainfall |
-0.61 |
0.14 |
0.09 |
Exp.Var |
1.51 |
1.27 |
|
Total Prp. |
0.3 |
0.25 |
Table 2 Non-rotated factor analysis between Culex genus and climatic variables during the period. Values in bold show association above ±0.23 and, in red, above ±0.70
The concentration of atmospheric CO2 fluctuates annually according to the period of the year, suggesting an influence on the density of this genus even with the opposite behavior with air temperature and rain. Water vapor is not very clear about its association with vectors.
The variability of atmospheric CO2 concentration may be associated with other meteorological variables, such as air temperature (see correlation matrix); therefore this association with Culex can be a “false” association when including the entire period related (night and day, wet and dry).
Culex density, including seasonal variability, can be explained by two different conditions, during the dry season there are significant positive associations with atmospheric CO2 concentrations, non-significant with rainfall and negative, non-significant associations with water vapor and significant with air temperature (Table 3).
Unrotated |
factor 1 dry |
factor 2 dry |
factor 1 wet |
factor 2 wet |
Culex |
-0.66 |
-0.38 |
-0.65 |
0.37 |
CO2 |
-0.91 |
-0.05 |
0.02 |
0.95 |
H2O |
0.55 |
0.33 |
-0.51 |
-0.41 |
tair |
0.92 |
-0.14 |
0.86 |
-0.35 |
rainfall |
-0.45 |
0.67 |
0.59 |
0.3 |
Exp.Var |
3.26 |
1.48 |
2.69 |
1.93 |
Total Prp. |
0.47 |
0.21 |
0.38 |
0.28 |
Table 3 Non-rotated seasonal variability during the rainy (wet) and dry (dry) seasons, presenting the analysis of factors for the genus Culex
During the rainy season, there is a positive, significant association with atmospheric CO2 concentration and non-significant with rainfall in the 2nd Factor and non-significant negative association with water vapor and air temperature. Therefore, air temperature always presents a negative association, suggesting that this genus tends to reduce its density during the hottest periods and, on the other hand, to be associated with the concentration of atmospheric CO2 in both periods.
Rain has a positive association during the dry season and a negative association during the rainy season, that is, too much or too little rain tends to affect the insect population. In summary, of the five campaigns, the population density of the Culex genus is positively associated with high relative humidity and negatively associated with the rainy season.
Although its physiology indicates that its eggs do not have much resistance to desiccation, this genus reacts very well to prolonged periods of drought, so the survival of the eggs may depend primarily on moisture rather than high amounts of rain. Theoretically, the concentration of atmospheric CO2 tends to significantly influence the temperature of the ecosystem, affecting the mosquito population throughout the period, as well as during the seasons (rainy and dry).
Statistical analysis between coquillettidia and principal component analysis (PCA)
The factor loadings obtained from the Factor Analysis of the genus Coquillettidia and environmental variables during the study period, without rotation (table 4), where in the 1st factor, which explains 29% of the variance, there is a significant negative association between the gender Coquillettidia and rain were not significant with air temperature and atmospheric CO2 concentration and positively with water vapor. In the 2nd factor, which explains 25% of the variance, there is a significant positive association of Coquillettidia with atmospheric CO2 concentration (-0.74) and a non-significant negative association with water vapor (0.63).
Unrotated |
Factor 1 |
Factor 2 |
R2 |
Coquilettidia |
-0.55 |
-0.51 |
0.09 |
CO2 |
0.25 |
-0.74 |
0.09 |
H2O |
-0.43 |
0.63 |
0.09 |
tair |
0.62 |
0.17 |
0.09 |
rainfall |
0.72 |
0.09 |
0.11 |
Exp.Var |
1.45 |
1.25 |
|
Total Prp. |
0.29 |
0.25 |
Table 4 Factor analysis where the Coquillettidia genus and climate variables are associated
Analyzing seasonal variability, the results show differences during the dry season (Table 5). The concentration of atmospheric CO2 (CO2) appeared in the 1st Factor during the dry season (with 44% explained variance) and in the 2nd Factor, during the rainy season (with 27% of variance), both factors with a significant positive association with the Coquillettidia genus. On the other hand, water vapor showed opposite association in 1st Factor for the dry season and no association in the rainy season. A similar behavior is shown by air temperature, suggesting that the hot and humid climate during the dry season decreases the population density of mosquito vectors for this genus.
Unrotated |
factor 1 |
factor 2 |
factor 1 |
factor 2 |
Coquilettidia |
-0.49 |
0.05 |
0.14 |
-0.45 |
CO2 |
-0.89 |
0.15 |
-0.24 |
-0.91 |
H2O |
0.49 |
-0.43 |
0.64 |
0.18 |
tair |
0.92 |
0.05 |
-0.76 |
0.55 |
rainfall |
-0.52 |
-0.6 |
-0.62 |
-0.18 |
Exp.Var |
3.09 |
1.37 |
2.42 |
1.89 |
Total Prp. |
0.44 |
0.2 |
0.35 |
0.27 |
Table 5 Seasonal variability during the rainy (wet) and dry (dry) seasons, showing the non-rotated factor analysis for the genus Coquillettidia
Rain shows a similar behavior, although with a positive association in the 1st Factor during the dry season, when the entire period is included, which is expected, showing similarity to the population density of the Culex genus.
During the five campaigns, the population density of the genus Coquillittedia decreased with heavy rainfall and high temperatures throughout the period. The amount of rainfall negatively affects the population density of this genus, although the latter association is more complex, presenting opposite behavior when seasonal differences are included. On the other hand, the concentration of atmospheric CO2 indicates an evident association throughout the period and when the seasons are different, although it may be associated with another variable, with the same explanation for the genus Culex.
In order to analyze the data series anomaly, factor analysis was applied not to the data, but to the difference between the mean values of the environmental and climatological data.
Factor analysis of culex anomalies
Factor analysis of the Culex genus as a function of normalized climatic variables without rotation throughout the study period (Table 6), where the 1st factor, which explains 32% of the variance, does not show any association of the Culex genus (- 0.09) with atmospheric CO2 concentration and climate variables. In the second factor, with 25% of the variance explained, there is a positive association of this type (0.74) with water vapor (0.55) and a negative association with rain (-0.54).
Unrotated |
Factor 1 |
Factor 2 |
R2 |
Culex |
-0.09 |
0.74 |
0.05 |
CO2 |
0.72 |
-0.2 |
0.15 |
H2O |
-0.45 |
0.55 |
0.08 |
Tair |
-0.74 |
-0.21 |
0.17 |
rainfall |
-0.56 |
-0.54 |
0.12 |
Exp.Var |
1.59 |
1.23 |
|
Total Prp. |
0.32 |
0.25 |
|
Table 6 Unrotated factor analysis between Culex genus and climatic variables during the period. Values in bold show association above ±0.23 and in red, above ±0.70
For this situation, the population density of this genus indicated an oscillation according to the fluctuation in the concentration of water vapor and rain, while the concentration of atmospheric CO2 has no impact, however it should be noted that during the dry season (April- October) and only in the daytime period, the Culex genus shows a strong positive association.
These results emphasize that the concentration of atmospheric CO2 can directly influence the atmosphere of the ecosystem, contributing to positively affect the population density of this genus, although this influence is during specific meteorological conditions.
From the above analysis, during the five campaigns, moderate rainfall events as well as low levels of water vapor tend to affect the Culex population density, theoretically decreasing it, as expected, as long as they directly prevent rain events.
Analysis of anomaly of the genus coquillettidia
The normalized analysis of the association between the genus Coquillettidia and the environmental variables for the study period (Table 7), where in the 1st factor, which explains 28% of the variance, there is a negative association of the genus (-0.67), not significant with air temperature (0.55) and significant with rain (0.76). In the 2nd factor, which explains 28% of the variance, there is no association with the mosquito.
Unrotated |
Factor 1 |
Factor 2 |
Communalities |
R2 |
|
Coquilettidia |
-0.67 |
0.19 |
0.45 |
0.49 |
0.05 |
CO2 |
-0.16 |
-0.78 |
0.03 |
0.63 |
0.15 |
H2O |
-0.17 |
0.7 |
0.03 |
0.52 |
0.06 |
Tair |
0.55 |
0.49 |
0.3 |
0.54 |
0.17 |
rainfall |
0.76 |
0.13 |
0.58 |
0.6 |
0.13 |
Exp.Var |
1.39 |
1.39 |
|||
Total Prp. |
0.28 |
0.28 |
|||
Table 7 Unrotated Factor Analysis and Commonalities associating the genus Coquillettidia, the climatic variables during the period. In bold, the association above ±0.40 and in red, greater than or equal to ±0.70
In summary, the atmospheric CO2 concentration anomaly has no clear effect, while air temperature and rainfall have a behavior opposite to population density. However, the genus Coquillettidia shows a positive association with the concentration of atmospheric CO2, with similar effects to the genus Culex (Tables 2 and 3). This result emphasizes again that the concentration of atmospheric CO2 can positively influence the population density of both genders.
During the five campaigns, moderate rainfall events also affect the population density of Coquillettidia, reducing it, having the same explanation given to the genus Culex.
Analysis of the variability of factor scores
The following figures show the variations in the score factor of the Factor Analysis performed for the population density of mosquitoes of the Culex and Coquillettidia genera.
It is possible to observe that there is a significant but low correlation (0.27) between the F2 score and the Culex genus (Figure 6) and both factors present a low correlation (0.23 and 0.34 respectively) for the Coquillettidia genus (Figure 7). It was observed through a factor analysis that the variability of the scores was statistically significant, albeit with low values, with the mosquito population density. Part of the variability in mosquito population density is not explained by the identified factors and may be related to other factors that are important in describing the variability in population density.
The global results clearly show the impacts of environmental variables on the population densities of the Culex and Coquillettidia genera with an emphasis on the eco-physiological behavior of both. The concentration of water vapor had different influences on the densities of the Culex and Coquillettidia genera, which are more controversial. Noting that the downward bias of this variable is, in particular, due to climate change that directly affects both genders, being particular in Culex, as it presents a greater explanation of the variance.
An expressive decadal variation in the behavior of the rains suggests that it significantly affects the hydrological cycle of the region, since the amount of rain negatively affects the population density of mosquitoes during the rainy season, due to the highly physical impact of raindrops on the insect. The opposite behavior occurs during the dry season, when the amount of rain available is quite relevant, causing a forcing on the insect's physiology.
Air temperature negatively affects both genders of mosquitoes who tend to prefer a more comfortable climate. Therefore, the climate change to hotter/drier and with rains, although more intense/sparse, tend to decrease the density of both genera, Culex and Coquillettidia.
Although the analysis of the anomalies presents an inconclusive result for the association of atmospheric CO2 concentration with mosquitoes, the results, in general, indicate the existence of a clear positive association between this variable and the population densities of both Culex and Coquillettidia. When, in particular, the diurnal and the seasons (dry and rainy) are analyzed separately, the positive association is even more evident. Therefore, the concentration of atmospheric CO2 and the population density of both genders of mosquitoes show an association that is very relevant due to the elevation of greenhouse gases and the global impact of warming on other variables, which demands further experimental research. with this association.
There is a clear trend of an annual increase in air temperature and amount of rainfall as well as a decrease in relative humidity during the last decades that also negatively affect mosquito populations. These results can be associated with the level of global warming, where the concentration of atmospheric CO2 plays an important role, emphasizing deeper studies of the impact of environmental variables that tend to affect Amazonian biodiversity.
None.
The author declares there is no conflcit of interest.

©2022 da, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.