International Journal of
eISSN: 2576-4454


Research Article Volume 6 Issue 6
1Institut de l’Environnement et de Recherches Agricoles, Département Gestion des Ressources Naturelles et Systèmes de Productions (INERA/GRN-SP), Bobo-Dioulasso, Burkina Faso
2Laboratoire Eaux Hydrosystèmes et Agriculture (LEHSA), Institut International d’Ingénierie de l’Eau et de l’Environnement (2iE), Ouagadougou, Burkina Faso
3West African Science Service Centre on Climate Change and Adapted Land Use (Wascal) - Institut National de l’Eau, Université d’Abomey Calavi, Benin, Burkina Faso
4Université de Fada N’Gourma au Burkina Faso, Burkina Faso
Correspondence: Dende Lushima Zacharie, Institut de l’Environnement et de Recherches Agricoles, Département Gestion des Ressources Naturelles et Systèmes de Productions (INERA/GRN-SP), Bobo-Dioulasso, Burkina Faso, Tel +22666514695
Received: December 17, 2022 | Published: December 30, 2022
Citation: Zacharie DL, Gaetan SES, Michelle CHM, et al. Suitability of urban river water for irrigation: the case of the Houet river in Burkina Faso. Int J Hydro. 2022;6(6):243-251. DOI: 10.15406/ijh.2022.06.00332
This study focused on the suitability of using an urban river for the irrigation of vegetable crops. The relevant urban river is Houet river located in the city of Bobo-Dioulasso (Burkina Faso) receives wastewater released by a wastewater treatment and purification unit (WWTP). To do this, a diagnosis of the functioning of WWTP and an assessment of the quality of the effluents released in the river were indeed carried out. The WWTP treats wastewater from industries, households and the percolating water from the treatment of the city's sewage sludge. Water released by the WWTP has a poor physico-chemical quality and high microbiological pollution. The urban river water suitability for irrigation was carried out by taking 8 water samples from the river sections at upstream and downstream parts of the WWTP junction. The physico-chemical (pH, EC, STD, Ca2+ , Mg2+ , K+ , Na+ , Cl-, HCO3- , PO43- , NO2- , NO3- , NH4+ ), microbiological (total coliform, fecal coliform, Escherichia coli and fecal Streptococci), trace metallic elements (Ni, Cr, Zn, Cu, Al, Fe and Mn) and indices such as sodium adsorption rate (SAR), percentage of soluble sodium (%Na), permeability index (PI), magnesium adsorption ratio (RAM) and Kelly ratio (RK) were analyzed and the results compared with WHO, FAO and USSL guidelines for irrigation water. The results show that the physico-chemical parameters are in line with the WHO and FAO standards for irrigation. However, the river water is reported to have medium salinity with a slight to moderate degree of restriction for EC and STD. The concentrations of ammonium (NH4+), manganese (Mn) and nickel (Ni) in the river’s water have values above standard reference for irrigation water. This suggests (i) overuse of nitrogenous fertilizers, (ii) industrial and anthropogenic discharges directly into the river, (iii) and an incomplete treatment process of water released by the WWTP. The presence of Total Coliforms, Fecal Coliforms, Escherichia coli and Fecal Streptococci at levels of around 104 to 109 CFU/100ml, above the FAO standard (2000 CFU/100ml), indicate that the river water is probably contaminated by viruses, parasites and other pathogenic bacteria of fecal origin. Houet river water is in excellent range for irrigation according to Wilcox diagram and in the C2 S1 range of medium to good quality according to the Riverside diagram. The SAR, %Na, PI, RAM and RK indices showed that the infiltration problem issue of the river water on irrigated soils is low before its junction with the WWTP and moderate after its junction. Overall, this river water is suitable for irrigation, but the presence of bacteria, ammonium and trace metallic elements require for the regional water and agricultural authorities to (i) pay particular attention to human activities in the vicinity of the river, (ii) improve the efficiency of the wastewater treatment process at the WWTP and (iii) more strictly control fertilizers’ uses by market gardeners. This will make it possible to preserve the quality of the river’ water for the irrigation of 175 hectares of market gardens products (lettuce, parsley, pepper, mint…) using a commercial urban outland to feed an urban population estimated of one million people.
Keywords: river water, purification unit, microbiology, chemical parameters, irrigation suitability
Freshwater is a prime natural resource, a precious commodity and one of the main constituents of the environment necessary for the continuity of life.1,2 The scarcity of freshwater resources (river water, lake water, etc.) and their contamination by poorly or badly treated wastewater are combined to a global context of increasing population and rapid urbanization for increasing number of water users in addition to climate change impacts; hence, a need for rational and efficient management of these water resources.3,4 In irrigated agriculture, irrigation water comes from (i) a river or lake1,5,6 (ii) boreholes/wells exploiting groundwater7–9 or (iii) a wastewater treatment unit.10–12 According to Acharya and al. (2020) the productivity of irrigated crops is linked to the quantity and quality of water used, which can have a significant influence on the agronomic and soil properties and on crops quality.1 Poor irrigation water quality can lead to toxicity, salinity, nutrient deficiencies for plant roots, soil flooding and other problems.13,14
In Burkina Faso, the urban river Houet in the second largest city (Bobo-Dioulasso) of the country is a source of socio-economic activity for the market gardeners who cultivate in its vicinity. It provides irrigation water for 1/3 of the city's urban and peri-urban gardens.15 Domestic discharges, land washing, wastewater discharges from industries and the city's wastewater treatment and purification unit (WWTP) are released into the river and very often without adequate treatment.16 The WWTP unit was built in 2008 to collect and treat domestic and industrial wastewater. Although it has improved hygiene and sanitation conditions in Bobo Dioulasso, WWTP (i) only takes in part of the domestic and industrial wastes17 (ii) and does seem and incomplete wastewater treatment and purification as it does not have any maturation basin.16,18,19 These situations present potential risks of pollution and contamination for the Houet river water by bacteriological and physico-chemical components. To this end, it appears crucial and prerequisite to understand and have good knowledge on the quality of the Houet river water used for cropping vegetables. The objective of this study is to examine the suitability of the Houet River receiving the WWTP pretreated wastewater for irrigation.
Overview of the Houet river
Houet river, is a perennial urban river that crosses the city of Bobo-Dioulasso from south to north, draining the northern sector of the city (Figure 1). It serves as an outland for the city's main rainwater collectors for multiple interests. In addition to the sacred catfish of Bobo-Dioulasso, it is exploited for the irrigated market garden crops fruit trees and others socio-economic activities (leaching, brickworks ...) for the riparian population.15,20 Market gardening contributes to 15% of the country's vegetable and fruit production.21 According to Robineau (2013), the total area of market gardening alongside the river is 175 ha.22 The city of Bobo-Dioulasso has an average annual rainfall and temperature fluctuating between 900 mm and 1200 mm, and between 25°C and 30°C respectively.17,21

Figure 1 Geographical location of Bobo-Dioulasso and overview of the Houet River.21
Methodology approach
In the present study, a diagnosis of the operating state of the WWTP and the physico-chemical and bacteriological quality of the discharged water was carried out. This was followed by a suitability assessment of the Houet river water for irrigating crops on market gardens. The diagnosis of the operational status of the WWTP consisted of a visual inspection of the unit compartments; i.e. the devices, the pre-treatment and treatment processes of the received and discharged wastewater and the management structure. The evaluation of the physico-chemical and microbiological quality consisted in:
These analyses covered pH, temperature, electrical conductivity (EC), total dissolved solids (TDS), calcium (Ca2+), magnesium (Mg2+), potassium (K+), Sodium (Na+), Chloride (Cl-), Bicarbonate (HCO3-), Ortho-phosphate (PO43-), Ammonium (NH4+), Nitrite (NO2-) and Nitrate (NO3-), Trace metallic elements like Nickel (Ni), Chromium (Cr), Zinc (Zn), Copper (Cu), Aluminum (Al), Iron (Fe) and Manganese (Mn) and bacteria (Total Coliforms (TC), Fecal Coliforms (FC), Escherichia coli (EC) and Fecal Streptococci (FST)). The physical parameters (pH, EC and STD) were measured in situ using an Aquaprobe AP-2000. The other parameters were measured in the Water-Hydro-Systems and Agriculture Laboratory of the 2iE Institute. Na+ and K+ were determined by the flame photometry method; HCO3- by acidimetry, NO3-, NH4+ and NO2- by the HACH 8039 cadmium reduction method; PO43- by the HACH 8114 PhosVer 3 method; Ca2+ and Mg2+ by the complexometric method; bacteria (CT, CF, EC and ST) by membrane filtration and trace metallic (Ni, Cr, Zn, Cu, Al, Fe and Mn) by the flame atomic absorption method. The river water suitability for irrigation was evaluated according to guidelines of the World Health Organization (WHO), the Food and Agriculture Organization of the United Nations (FAO) and the United States Salinity Laboratory (USSL).23–29 Calculation of water quality indices from the equations below was also used to estimate the river water suitability for irrigation.1,30–34
Eq. 1
Eq. 2
Eq. 3
Eq. 4
Eq. 5
Where SAR is the Sodium Adsorption Ratio, %Na is the percentage of soluble sodium, PI is the Permeability Index, RAM is the Magnesium Adsorption Ratio and RK is the Kelly Ratio. The units of all ions are in meq/l (milli equivalent per liter). The Wilcox (%Na = f(EC)) and Riverside (SAR=f(EC)) plots of the USSL were used on the basis of SAR, %Na and EC values to indicate the suitability of waters for irrigation.35
Diagnosis of the water treatment and purification unit
Operational status of the WWTP
Consisting of a wastewater treatment system and a fecal sludge treatment system, the WWTP receives a part of the domestic and industrial wastewater of Bobo Dioulasso.18,19 The connected plants to it are agri-food, including the beer company of Burkina Faso (Brakina), the new oil and soap company (SN-Citec), the agricultural products trading company (AgriFaso) and the refrigerated slaughterhouse. Unfortunately, only two of these plants have a pre-treatment system for their wastewater before they are released into the WWTP sewage drainage channel WWTP17 (Figure 3). The WWTP is a microphyte lagoon system, in which wastewater flows through one or more shallow basins, without oxygen supply and relying on the self-cleaning power of microorganisms combined with algae. It consists of a medium bar screen, a grit/oil separator, a Parshall flow measurement channel, two series of drying beds and two anaerobic tanks in parallel and both in series with the facultative basin. In addition to wastewater and industrial effluents, the WWTP receives percolation water from fecal sludge treatment system17 (Figure 4a). The present study reveals that the wastewater treatment at the WWTP is deficient. It was observed that (i) pink color water at the facultative basin meaning of the presence of red algae (rhodophyceae) and purple bacteria blooming under anaerobic conditions with sulfate reduction (Figure 4b), (ii) a lot of sludge on the surface of the facultative basin which blocks the penetration of solar radiation. This situation limits the photosynthetic activity and consequently favors algal concentration however necessary for the degradation of pollution, (iii) the presence of solid waste in the anaerobic basin due to the poor operation of the screening and grit removal devices (Figure 4c and d); (iv) an unpleasant smell due to the bacterial reduction of sulfates into hydrogen sulfide. The WWTP does not have a maturation basin, a situation which doesn’t allow for the removal of pathogens, nutrients and reduction of biodegradable organic matter.16,18,36
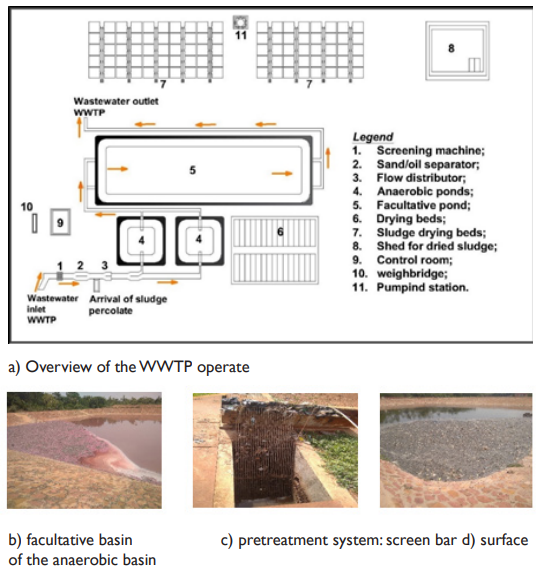
Figure 4 Diagnosis of the operate of wastewater treatment and purification unit. a) Overview of WWTP operation, b) Facultative basin with a pinkish color of the water on its surface, c) Solid waste indicating faulty operation of the screen, d) Pathogens on the surface of the anaerobic basin.
Organizational structure of the WWTP
The National Office for Water and Sanitation (ONEA) is country institution in charge of water and sanitation throughout Burkina Faso. In line with its missions, ONEA has set up the Bobo-Dioulasso treatment and purification unit, which is managed by the Bobo Regional Directorate (DRB). At the regional level, the WWTP is under the supervision of the office dedicated to collective sanitation, whose main mission is to manage industrial or autonomous collective sanitation facilities for the evacuation of wastewater and excreta. The WWTP has only one technician and two workers responsible for the operation and general maintenance of the wastewater treatment system and the fecal sludge treatment system. Unfortunately, these agents only carry out once a week the removal of garbage at the screen bar, the percolate fecal sludge and wastewater intended for the physico-chemical analyzes.
Physical and organic pollution parameters
Figures 5 and 6 show the variation, during the dry (April) and rainy (July) seasons from 2011 to 2021 of the pH and electrical conductivity of the wastewater pretreated and released by the WWTP. The pH ranges from 7.7 to 8.8 in the dry season and 7.8 to 8.8 in the rainy season. Electrical conductivity values range from 2035.5 to 8682 μS/cm in the dry season and 366.2 to 3210 μS/cm in the wet season. All values of EC in the dry season and 40% in rainy season are above of the references requirements in OMS which is 2000 μS/cm.
Organic pollution parameters
Figure 7, 8 and 9 show from 2011 to 2021, the variation of the concentration of organic pollution parameters (TSS, COD and BDO5) of treated wastewater and released at the outlet of the WWTP. The TSS shows values ranging from 180.6 to 1228.5 mg/l in the dry season and 94 to 827 mg/l during the rainy season, which are higher than the WHO standards (150mg/l) except for the year 2014 and 2021 where the value is lower than the standard. COD shows concentrations range from 98.4 to 1677.3 mg/l in the dry season and 187.8 to 1532.7 mg/l during the rainy season, which are also higher than the WHO required concentration which is 50 mg/L. BOD5 shows concentrations ranges from 120 to 533.4 mg/l in the dry season and 60 to 177.8 mg/l in the wet season. The BOD5 concentrations are all above the WHO discharge standard of 40 mg/l.
Chemical and microbiological parameters
The characteristics of the chemical, bacteriological and trace metallic elements of the wastewater at the outlet of the WWTP are presented in Table 1. The results are for samples taken in 2021 during the rainy season (July). It is shown that the wastewater released at the outlet by the WWTP has values of Calcium (Ca2+) and Magnesium (Mg2+) lower than the WHO recommendations while the ortho-phosphate (PO43-), Ammonium (NH4+), Nitrite (NO2-), Sodium (Na+), Bicarbonate (HCO3-) and Chloride (Cl-) are parameters with very high values in relation to established standards. For the metallic trace elements like Zinc (Zn), Nickel (Ni) and Manganese (Mn), values comply with the WHO established standards except for Manganese (Mn). The presence of total coliforms (TC), fecal coliforms (FC), Escherichia coli (EC) and fecal Streptococci (FSC) indicate a high level of fecal contamination of the water released by the WWTP. The results of the physico-chemical parameters of wastewater at the outlet of the WWTP are obviously higher for almost all parameters analyzed, with differences ranging from a factor of 1.75 for EC to 20 for SO42- and 39 for manganese in relation to the WHO wastewater requirement for irrigation (Table 1). As for the microbiological elements, the concentrations are exceptional. They are 109 and 108 respectively for fecal coliforms and fecal streptococci compared to admitted WHO reference concentration of 103 for both fecal types. These high concentrations of physico-chemical and microbiological elements at the inlet of the bar screen of the WWTP are normal and originate from the nature and quality of the effluents of the 06 plants connected to the WWTP wastewater drainage network.
|
Parameters |
Inlet of WWTP |
Outlet of WWTP |
Abatement rate (%) |
OMS Requirement |
|
pH |
8,93 |
8,16 |
- |
6,4 – 10,5 |
|
CE (µs/cm) |
3515 |
1753 |
50 |
2000 |
|
STD (mg/l) |
2284 |
1139 |
50 |
- |
|
MES (mg/l) |
268 |
94 |
65 |
150 |
|
DC0 (mg O2 /l) |
1109 |
505 |
54 |
150 |
|
DBO5 (mg O2 /l) |
380 |
60 |
84 |
50 |
|
NO3- (mg/L) |
140 |
85 |
39 |
50 |
|
NO2- (mg/L) |
1,3 |
1,2 |
8 |
1 |
|
NH4+ (mg/l) |
72 |
20 |
72 |
1 |
|
PO43- (mg/l) |
77 |
73 |
5 |
5 |
|
K+ (mg/l) |
33 |
26 |
21 |
- |
|
SO42- (mg/l) |
4900 |
1100 |
78 |
250 |
|
Ca2+ (mg/l) |
28 |
21,6 |
23 |
500 |
|
Mg2+ (mg/l) |
6,48 |
2,64 |
59 |
200 |
|
Na2+ (mg/l) |
795 |
350 |
56 |
300 |
|
Cl- (mg/l) |
460,51 |
280,31 |
39 |
- |
|
HCO3-(mg/l) |
1201 |
662 |
45 |
- |
|
Cd (mg/l) |
0 |
0 |
- |
0,01 |
|
Pb (mg/l) |
0 |
0 |
- |
5 |
|
Mn (mg/l) |
7,69 |
6,72 |
13 |
0,2 |
|
Zn (mg/l) |
0,13 |
0,11 |
15 |
2 |
|
Ni (mg/l) |
0,12 |
0,04 |
67 |
0,2 |
|
Cr (mg/l) |
0 |
0 |
- |
0,1 |
|
Fe (mg/l) |
0,63 |
0,68 |
- |
5 |
|
Cu (mg/l) |
0 |
0 |
- |
0,2 |
|
Total coliforms (UFC/100ml) |
1,97x1010 |
1,74x1010 |
12 |
- |
|
Fecal Coliforms (UFC/100ml) |
1,10x109 |
5x106 |
99,5 |
2x103 |
|
Escherichia coli (UFC/100ml) |
5,4x108 |
2x106 |
99,6 |
- |
|
Fecal streptococci (UFC/100ml) |
9,64x108 |
3,2x108 |
66,8 |
2x103 |
Table 1 Results of microbiological and physico-chemical parameters of WWTP
The wastewater treatment realizing by the WWTP does not lead to release of good quality water which scrupulously respects WHO water requirements for irrigation, even though if it allows decreasing in the concentrations of physico-chemical, microbiological and TME parameters. This is justified by breakdown rate in Table 1, of 65% for TSS, 84% for BOD, 72% for NH4+, 78% for SO42-, 67% for Ni, 99.5% for fecal coliforms, 99.6% for Escherichia coli and 66.8% for fecal Streptococci. The results are in line with those obtained by Onifida (2011) in the dry season at the WWTP and Soumbougma and al. (2020) in the wet season at the same WWTP Table 1.18,19
The poor performance of the treatment unit is explained by the poor quality of the effluents of the plants connected to the WWTP drainage network. The poor quality of pretreated wastewater used in downstream of Houet river for irrigation can have a negative impact on the vegetable’s productions.
Results of microbiological and physico-chemical parameters of Houet river water and suitability for irrigation
Physico-chemical and microbiological analyzed results of Houet river samples are recorded in Table 2.
Physico-chemical parameters
The results of the physico-chemical and microbiology parameters analyses are reported in Table 2. Physical parameters (T, pH, EC, STD) show values below the WHO and FAO requirements for irrigation water. The temperature of the river water ranges from 27.7°C to 29.93°C. The pH is slightly neutral to basic, ranging from 7.12 to 7.73. The electrical conductivity is between 416 μs/cm and 604 μs/cm. The TDS values vary between 270 mg/l and 392 mg/l. EC and TDS are indicative of the presence of salts in the water, with EC values below 700 mg/l indicating poorly mineralized water with no degree of restriction on the use of these waters for irrigation.25
The analysis of nitrogenous compounds shows concentrations ranging from 2.5 mg/l to 12.4 mg/l; well below WHO reference value of 50 mg/l. According to FAO (2003), when nitrate concentrations in water are ranging between 5 to 30 mg/l, waters are considered having light to moderate degree of restriction of use for irrigation.25 This situation would correspond to all 07 water samples of the Houet river except for point 8 located at downstream of river after its junction with WWTP where concentrations are around 2.5 mg/l. The nitrite concentrations are low and range from 0.003 mg/l to 0.012 mg/l, they also remain in line with WHO and FAO requirements for irrigation as the limit is fixed at 1 mg/l.
On the other hand, the ammonium concentrations of the Houet river vary from 1.2 mg/l to 7.6 mg/l, they are higher than the WHO standards which fix a threshold value of 1 mg/l. The Figure 10 shows a correlation of NH4+ ions with Na2+ (r=0.83) and HCO3- (r=0.81). These correlated NH4+, Na2+, and HCO3- ions; therefore, have the same anthropogenic origin. Indeed, the high values of ammonium, bicarbonate and sodium in river water can be due to the leaching of cultivated soils, domestic wastewater released and the industrial wastewater discharges from agri-food plants (Brakina, SN-Citec, AgriFaso and the slaughterhouse) using ammonium bicarbonate (NH4 HCO3) whandher or not in association with sodium bicarbonate (NaHCO3) as a food additive.37–40 According to Leghari et al. (2016); excessive supply of nitrogen fertilizer to the plant leads to plant overgrowth which delays fruit maturity and deteriorates fruit quality.41 The analysis of the phosphorus results shows PO43- concentrations ranges of 0.5 mg/l to 1.06 mg/L below the WHO requirement of 5 mg/l. The concentrations of potassium, calcium, magnesium and bicarbonates have value ranges of respectively 16 mg/l to 19.2 mg/l; 37.6 mg/l to 43.2 mg/l; 0.72 mg/l to 5.04 mg/l, and 118.34 mg/l to 204.96 mg/l. These values remain within recommended concentrations by WHO for irrigation water. The Sodium and Chlorides present values that are respectively ranging from 27 mg/l to 79.5 mg/l (Na+) and 60.07 mg/l to 74.08 mg/l (Cl-). According to FAO and LSUS standards for irrigation water, the river water would have no restrictions for irrigation with respect to Cl-, but its uses would be light to moderate for Na+ for the sections of the river located downstream of its junction with the WWTP.
Trace metal elements (TME)
The concentrations of TMEs are shown in the Table 2. They vary from 5.52 mg/l to 7.92 mg/l for manganese, 0.03 mg/l to 0.08 mg/l for zinc, zero to 0.25 mg/l for nickel and 0.03 mg/l to 0.97 mg/l for iron. Exception of zinc, iron, manganese and nickel have concentrations above the FAO guide values for irrigation water. A strong correlation is observed on Figure 11 between manganese Mn and potassium K+ (r = 0.84) and calcium Ca2+ (r = 0.87) ions. These relationships could mean that these elements have the same sources of mineralization. The presence of these elements in the river could be due to leaching of clay minerals from the soil or agricultural land, groundwater-river exchanges, domestic wastewater discharges42,43 and fertilizers used by irrigators in the vicinity of the river. Similarly, the high values of Mn and Ni in the river could be due to the pH of the water, the geological and pedological formation of the area, the discharge of industrial and anthropic effluents.44–47 The market garden soils in the vicinity of the river are of hydromorphic ferralitic type on armourstone. The oxidation of such soils in the presence of alkaline or acid water and microorganisms would favor the precipitation of Mn and Ni.48 In addition, the presence of manganese and nickel in the water could be due to the impact of industrial and anthropogenic discharges directly into the river.45-47 Figures 10 & 11
Microbiological parameters
The results of the microbiological analyses are reported in Table 2 which reveals significant levels of Total Coliforms, Fecal Coliforms, Escherichia coli, Fecal Streptococci the concentrations range from 104 to 109 CFU/100ml and higher than the recommended values of FAO (2000 CFU/100ml). These waters would therefore not be suitable for irrigation and their presence suggests that the river water is likely to be contaminated by viruses, parasites and other pathogenic bacteria of fecal origin.49–51 Environmental conditions, such as the household waste, industrial and anthropogenic wastewater discharges are the cause of the high level of fecal contamination of rivers water by pathogenic bacteria.49–52
Suitability of river water for irrigationn
SAR, %NA, IP, RAM and RK indices
The suitability of the Houet river for irrigation was estimated using SAR calculation method, %Na, IP, RAM and RK indices (Table 2). SAR and %Na are important parameters in determining suitability of irrigation water. high concentrations of sodium in water can affect soil permeability and cause infiltration problems.9 The SAR values range from 1.03 to 3.27 and the %Na has values ranges of 38.45% to 63.33%. Based on the SAR values, LSUS considers these waters to be very good, suitable for all types of crops and without any soil disturbance. For %Na values, Houet river water is considered good at point 1, average from point 2 to 5 and doubtful from point 6 to 8. The PI of the river water shows values range of 68.47% to 93.06%; corresponding to low and good permeability category at point 1 and moderate for point 2 to point 8. The SAR, %Na and PI values vary in the river water. This can be explained by external water inputs and probably minerals coming from leaching of agricultural soils. The permeability problem occurs when the normal infiltration rate of the soil is significantly reduced and this hampers the supply of moisture to the crops and also its contents in sodium, calcium and magnesium.53 The magnesium absorption ratio (MAR) values range from 2.35% to 14.89% and are well below the limit value of 50%.28,54 The Kelly ratio (RK) considers water to be suitable if RK<1 and unsuitable if RK> 1.28,33,55 The RK of Houet river ranges from 0.46 to 1.54. According to the established criteria, the river water is considered suitable for point 1 to point 5 located before the junction with WWTP and unsuitable for point 6 to point 8 located after the junction with WWTP. The wastewater released by WWTP rich in physico-chemical parameters could explained, the high concentrations of Sodium compared to calcium and magnesium.54,56
The Wilcox and riverside diagram
The Wilcox diagram classifies all river waters as excellent for irrigation (Figure 12). The Riverside diagram (Figure 13) identifies the river water for irrigation in the C2 S1 class (250 < C2 > 750 μS/cm and 0 < S1 > 14) of average to good quality for irrigation. According to the United States Salinity Laboratory (USSL), they can be used with caution in poorly drained heavy soils and for sensitive plants (fruit trees). Figure 12,13.
The analysis of physico-chemical parameters of the Houet river presented concentrations in line with WHO, FAO and USSL requirements; except for ammonium which concentrations are above WHO and FAO standards. The analysis of temperature to assess surface water quality is important as it affects biological processes in aquatic systems and can lead to accidents especially on young plants in case of high or low temperature.3,57 The temperature values are similar to those found by Tampo and al. (2015) in the Zio river in Togo.58 The pH of the Houet river is neutral to basic and in line with FAO (2003) requirement which suggests that the water pH for irrigation be neutral to basic.25 It plays a key role in nutrients use by plants and extreme value of pH can reduce or even completely inhibit crop growth.19,59 These pH values are close to those found by Buhungu and al. (2018) on the Kinyankonge river, a tributary of Lake Tanganyika which receives like the Kou river, domestic discharges, soils leaching minerals, discharges from soap factory and those from a Buterere wastewater treatment unit (WWTP).3 EC and STD are important parameters for determining water quality for irrigation.11,28,60 According to those parameters, Houet river has a medium salinity with slight to moderate restrictions.
The analyzed mineral and chemical elements (Ca2+, Mg2+, K+, Na+, Cl-, HCO3-, PO43- , NO2-, NO3- , NH4+), contribute in one way to another to plant growth.11,53,57,59,61,62 These include macronutrients such as nitrogen compounds, phosphorus and potassium, which contribute to plant tissues growth, roots development, photosynthesis and protein synthesis.25,62,63 The presence of these elements in water must be controlled as at high concentrations they can hamper crops growth. The variations in Ca2+, Mg2+, K+, Na+ are similar to those found by Senou and al. (2020) in the Houet River during the dry season of 2012.64 The concentrations of Cl, Mg, Na and K are similar to those found in the dry season on the Massili River which is the main receptacle of almost all industrial wastewater (treated or not) from the city of Ouagadougou. On the other hand, the concentrations of Cl-, HCO3-, PO43- , NO2-, NO3- and NH4+, are similar to those reported by Nahli and al. (2016) on the Oeud Hassar river which receives also wastewater treated by a WWTP (the Médiouna WWTP), the rural wastewater associated with sparse solid household wastes from the locality of Sidi Hajjaj and Sidi Brahim as well as the leaching of agricultural lands.65
The results of the trace metal elements analysis (Ni, Zn, Fe and Mn) show high concentrations of iron, manganese and nickel above FAO water requirement for irrigation. TMEs are also important for the plants and require attention because at high concentration they can affect plants’ metabolism, mainly in enzymatic reactions.57,65–67 The results obtained are close to those obtained by Ahoussi & Yapo (2021) but lower than those of Chen et al. (2021) on surface waters in Nanjing (China) and Pienaar et al. (2020) in the Marico River in South Africa draining effluents from several industries.68 The microbiological analysis reflects a significant presence of bacteria (Total Coliforms, Fecal Coliforms, Escherichia coli, Fecal Streptococci) above FAO standard. These values are close to those obtained by Dianou et al. (2011) on the Sourou rivers, Mouhoun, Debe and Gana valley in Burkina Faso.69 The use of this water for irrigation can lead to plants contamination by micro-organisms during their growth or harvesting.62,70,71 From the indices calculation results (SAR, %Na, RAM, IP and RK), the infiltration problem is a little concern at upstream part of the junction with the WWTP and moderate at the downstream part. Based on the values of EC, %Na and SAR, the Houet river water is excellent for irrigation. These results differ from those of Senou et al. (2020) in the dry season who found SAR values of 9, indicating an unsuitable water for irrigation.64 This difference highlights the importance of the dilution phenomena that take place in the Houet River during the rainy season due to the contribution of rainwater, even after mixing with the wastewater from the WWTP which is polluted.18,19 The Houet river water could contribute to improve the productivity of market gardening but regarding to anthropic and agricultural activities in addition to the WWTP wastewater release, it is recommended to regularly monitor the river.
The study carried out on the Houet river has made it possible to evaluate the suitability for irrigation even that it receives wastewater from WWTP. The results showed that the physico-chemical parameters (pH, EC, STD, Ca2+ , Mg2+ , K+ , Na+ , Cl- , HCO3- , PO43- , NO2- , NO3- and NH4+); microbiological (total coliforms, fecal coliforms, Escherichia coli and fecal Streptococci), trace metals (Ni, Cr, Zn, Cu, Al, Fe and Mn) and other indices SAR (Sodium Adsorption Rate), %Na (Percentage of soluble sodium), IP (Permeability Index), RAM (Magnesium Adsorption Ratio) and RK (Kelly Ratio) are respectively good physicochemical quality, low trace metal pollution and high microbiological pollution. The salinity concern is average with a slight to moderate degree of restriction. The results of this study are expected to be carefully used for safeguarding the quality of the Houet river, located in a city with high agricultural activity, a growing population and increasing industrialization.
None.
The author declares that there are no conflicts of interest.

©2022 Zacharie, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.