Journal of
eISSN: 2373-6437


Research Article Volume 15 Issue 6
1University Clinic for Traumatology, Orthopedics, Anesthesia, Intensive Care and Emergency center, Ss. Cyril and Methodius University, Republic of North Macedonia
2Department of Doctoral studies, Faculty of Medicine, Ss. Cyril and Methodius University, Republic of North Macedonia
Correspondence: Aleksandar Kishman, University Clinic for traumatology, orthopedics, anesthesia and intensive care and emergency center, Ss. Cyril and Methodius University, Skopje, Republic of North Macedonia
Received: September 11, 2023 | Published: November 17, 2023
Citation: Kishman A, Sholjakova M, Kartalov A, et al. Comparative study of the effects of dexmedetomidin and midazolam on respiration and hemodynamics used during facial surgery. J Anesth Crit Care Open Access. 2023;15(6):167-170. DOI: 10.15406/jaccoa.2023.15.00574
Background: The interventions in facial surgery are specific, short, and very painful. The place of the surgery can compromise a patient’s airway. Surgeons prefer to give local infiltrative anesthetics, but during the surgery patients are nervous, stressed, restless and ask for drugs to be asleep/ask for sleep medication. Dexmedetomidine (Dex) as an agent for analgosedation can meet the needs of those patients. Dex is an alpha 2 adrenergic agonist with sedative, anxiolytic and analgesic properties; Midazolam (Mdz) is the most often used drug for classical sedation, a drug from the benzodiazepine group.
Aims: The aim of this study was to compare the effects on circulation and respiration of two drugs, dexmedetomidine (Dex) and midazolam (Mdz). Blood pressure, Puls/min, respiration/min, SpO2, incidents of bradycardia, hypotension, hypoxia, and other complications were measured and compared in the two groups.
Material and methods: Sixty patients for facial surgery who met the inclusion criteria were enrolled in the study. Due to a computed choice/option, patients were allocated to be sedated with Dex (n=30) or Mdz (n=30). After signing a written consent for inclusion in the study, prior to surgery all patients got two venous lines and were preoperatively monitored. Patients of Dex group received a bolus of Dex 1 mcg/kg given in 10 minutes. The sedation was maintained with an additional dose of Dex of 0.5 mcg/kg/h, which was disconnected at the end of the surgery. Patients of Mdz group received a bolus of Mdz of 0.03 mg/kg followed by an infusion of Mdz of 0.2 mg/kg/h, which was disconnected at the end of the surgery. The vital signs (ECG, BP, P/min, Res/min, SpO2, ETCO2, BIS) were monitored and noted on 5-minute intervals.
Results: The obtained results were statistically analyzed. Demographic data showed homogeneity between the groups. All patients prior to procedural sedation got local infiltrative anesthesia with lidocaine 1%. There was insignificant difference in duration of the surgical procedure between the groups (p>0.05) and prolonged induction to sedation in the Dex group (10.6±2.7* vs. 1.9±1.7); p=0.01. Blood pressure of patients in the Dex group showed a significant decrease and was lower than that in the Mdz group (p=0.05). Also, P/min in the Dex group was lower than in the Mdz group (74.45±14.84*vs. 84.13±12.88) p=0.03. The results from the monitored respiration showed a statistically significant decrease in respiration/minute in the Mdz group (p=0.05) and decrease in SpO2, (p=0.02).
Conclusion: We found that Dex, used as an agent for analgosedation for facial surgery, is a safe drug providing hypotension and mild bradycardia which are easy for treatment with vasoconstrictors and a sedation without effects on respiration. Patients in the Dex group were more comfortable, and their satisfaction was higher than in patients in the Mdz group.
Keywords: dexmedetomidine, midazolam, procedural sedation, respiration, mean arterial blood pressure
The contemporary views on safe anesthesia imply good analgesia and patient comfort during surgical interventions.1 In that context, anesthesiology today strives for increased patient care of particular interest to anesthesiologists are short and very painful surgical interventions, such as interventions in plastic and reconstructive surgery, which usually involve a one-day hospital stay of patients. For this pathology, the choice of right analgesia and sedation is crucial for a good outcome (of the intervention). Perioperative sedation as an anesthetic technique is used to calm patients before and during a surgical procedure. According to ASA (American Society of Anesthesiologists), sedation is classified into 4 degrees of depth (Table 1).
Minimal sedation (Anxiolysis) |
Moderate sedation and/or analgesia |
Conscious or (‘procedural sedation'-PS) |
Deep sedation and/or analgesia ('analgosedation'-AS) |
General anesthesia |
Table 1 ASA ranking of degrees of sedation
Midazolam (Mdz) and Dexmedetomidine (Dex) are sedative agents commonly used for perioperative sedation and analgesia, and additionally fentanyl for achieving analgesia.2 Midazolam is a classic sedative agent, which usage for PS is popular. It is a benzodiazepine used preoperatively for premedication, perioperatively for PS, as an adjuvant to total venous anesthesia (TIVA) or as one of the agents for multimodal analgesia. Dexmedetomidine (Dex) is an alpha2-adrenergic agonist, a relatively new drug, which was primarily registered and used as a sedation drug in intensive care units (ICUs).3 It has sedative, anxiolytic, and analgesic properties. In recent years, due to its sedative and analgesic properties, as well as due to the possibility of saving the use of opioids, its use during the perioperative period has been increasing.4 So, it can be safely used during all anesthesia and surgical interventions. The biggest advantage is its application as an agent for procedural sedation, as an adjuvant to regional blocks and during general anesthesia.5 Sedative effects are due to inhibition of neuronal transmission in the locus coeruleus (LC) in the brainstem, from where a state of unconsciousness, similar to natural sleep, with a unique possibility of easy awakening and cooperativeness is induced. The analgesic effect is due to a direct central action on the LC. The application of Dex for sedation or as an adjuvant to anesthesia is in the form of IV infusion. A bolus dose is given in the first 10-20 minutes, and then it is continued continuously at a maintenance dose.6 Hypotension, hypertension, dry mouth, nausea, muscle weakness, arrhythmia, AV block, acidosis with hyperkalemia may occur after its application. It is considered that there are no absolute contraindications for its use. In the literature, there is a lack of data on perioperative use of Dex as an adjuvant to regional and general anesthesia, which was the initial motive to conduct this study.
The primary objective of this study was to measure and compare the effects on circulation and respiration of two drugs, dexmedetomidine (Dex) and midazolam (Mdz) during plastic and reconstructive surgery, and to measure and the second objective to compare the incidence of bradycardia, hypotension, hypoxia, and circulatory or respiratory complications in the two groups of patients.
This was a prospective controlled randomized study, including 60 patients. The study was realized in the period from 15.09.2022 to 1.09.2023, at the Department of Anesthesia for plastic and reconstructive surgery at the University Clinic for Traumatology, Orthopedics, Anesthesia, Reanimation, Intensive Care and Urgent/Emergency Center (TOARILUC) at the 'Mother Teresa' Clinical Center - Skopje, and at the University Clinic for Plastic and Reconstructive Surgery, Skopje, Republic of North Macedonia. After permission from the Institutional ethics committee and written consent obtained from the patients, the study was started. Patients were randomly selected, respecting the set of selection and inclusion criteria, and according to the reception by the surgeon. Patients were randomized to receive a perioperative infusion of either Dex (n=30) or midazolam (n=30).
Inclusion criteria
Exclusion criteria
Before introduction to sedation, patients were randomized into two groups: Group Mdz (n=30) – these were patients who received iv midazolam 0.04 mg/kg followed as needed by fentanyl 1 ml or propofol 0.09 mg/kg/h; Group Dex (n=30) –patients in one venous line got an initial bolus dose for sedation induction with Dex at a speed of 1 mcg/kg, for 10 min, which was continued at a maintenance dose of 0.5 mcg/kg/h. Intraoperative hemodynamic and respiratory markers for safe anesthesia were monitored and measured, as well as the amount of the used opioids. All patients from both groups were monitored perioperatively, and vital parameters were measured every 5 minutes: BP and pulse/min, heart action was continuously monitored through ECG, pulse oximetry, capnometry (while maintaining ETCO2 at the value of 40 mmHg) and BIS spectrometry for depth of sedation which was maintained between 40-60, respiratory rate and SpO2. Also, changes in hemodynamics and use of vasoactive amines and sympathomimetics were monitored along with the appearance of perioperative pain in both groups (Mdz and Dex), as well as the amount of intraoperatively added fentanyl.
The results obtained in this study are shown in the following tables (Table 2). Groups were homogeneous, with similar characteristics. There was no significant difference regarding age and gender, body weight and premedication (the amount and type) that all subjects received as early morning oral premedication. Introduction to sedation (Table 3) was significantly longer when using Dex. The length of surgery in both groups was comparable with no significant differences. The duration of postoperative drowsiness and sedation was longer in the midazolam group. In both groups, perioperative sedation was adjuvant to regional blocks with no significant difference in groups 3:4, or in 10% versus 12% in the Dex group. In the midazolam group, additional analgesia with fentanyl was required in 4 (12%) patients.
|
Group |
Age (years) |
Sex (m/f) |
Body weight (kg) |
Premedication diazepam mg |
|
Midazolam (n=30) |
52.2±4,3 |
18/10 |
70.2±7.4 |
4.3±0.1 |
|
Dexmedetomidine (n=30) |
55.03±16 |
17/11 |
72.6±7.6 |
4.4±0.4 |
|
p |
0.53 |
NS |
0.35 |
NS |
Table 2 Demographic data for midazolam and dexmedetomidine groups (M±SD)
P<0.05 significant differences
|
Group (min) |
Duration of introduction |
Duration of surgery |
Postoperative sedation |
Block N/% |
|
Midazolam (n=30) |
1.9±1.7 |
67.6±27.9 |
13.1±4* |
3/10% |
|
Dexmedetomidine (n=30) |
10.6±2.7* |
60.3±23.07 |
5.6±1.5* |
4/12% |
|
p |
0.01* |
NS |
0.001* |
NS |
Table 3 Duration of introduction, length of surgery and sedation (M±SD)
P<0.05 significant differences
Examination of the markers of safe anesthesia sedation and analgesia measured 20 minutes after the incision, showed that the use of Dex reduced the mean arterial pressure (MAP) and caused mild bradycardia immediately after the introduction. Midazolam led to a decreased respiratory rate and a fall in pSO2 (Table 4).
|
Group |
MAP (mmHg) |
Pulse/min |
Respiration/min |
pSO2 |
|
Midazolam (n=30) |
101.43±16.89 |
84.13±12.88 |
11.3±1.2* |
95.4±1.6* |
|
Dexmedetomidine (n=30) |
91.77±15.58* |
74.45±14.84* |
14.1±1.1 |
98.5±0.7 |
|
p |
0.05 |
0.03 |
0.05 |
0.02 |
Table 4 Perioperative markers for safe sedation and analgesia (M±SD)
P<0.05 significant. MAP, mean arterial pressure; P, pulse; R, respiration; pSO2, saturation of the blood with oxygen.
On Table 5 are presented the values of the Mean Arterial blood Pressure (MAP) in the timing 1, 2, 3,4, and 5. They were compared to the preoperative values (M1). Midazolam produced a significant decrease of the MAP in the time 3, - 5 minutes after the incision. The surgical procedure and persistent pain increased the MAP (p>0.05), but it decreased at the end of surgery near preoperative values.
|
Midazolam |
No |
Mean |
Conf: -95% |
Conf: +95% |
min |
max |
SD ± |
p |
|
MAP 1 |
30 |
104.8 |
0.124 |
3.881 |
90 |
126 |
10.84 |
|
|
MAP 2 |
30 |
100.1 |
0.108 |
3.385 |
87 |
125 |
9.46 |
0.1 |
|
MAP 3 |
30 |
93.033 |
0.032 |
1.014 |
87 |
98 |
2.83 |
0.002* |
|
MAP 4 |
30 |
100.233 |
0.098 |
3.841 |
90 |
123 |
8.6 |
0.07 |
|
MAP 5 |
30 |
98.7 |
0.056 |
1.768 |
92 |
112 |
4.94 |
0.007* |
|
Dexmedetomidine |
No |
Mean |
Conf: -95% |
Conf: +95% |
min |
max |
SD ± |
|
|
MAP 1 |
30 |
100.933 |
0.144 |
4.475 |
80 |
127 |
12.522 |
|
|
MAP 2 |
30 |
78.866 |
0.147 |
4.557 |
57 |
98 |
12.754 |
0.0006* |
|
MAP 3 |
30 |
81.733 |
0.106 |
3.286 |
65 |
97 |
9.195 |
0.0009* |
|
MAP 4 |
30 |
91.1 |
0.124 |
3.841 |
70 |
115 |
10.749 |
0.001* |
|
MAP 5 |
30 |
96.5 |
0.114 |
3.539 |
73 |
116 |
9.905 |
0.134 |
Table 5 Peroperative mean arterial blood pressure in the study groups (M±SD)
MAP, mean arterial blood pressure; M1, before surgery; M2, after induction; M3, 5 min after incision; Mean4, 20 min surgery; Mean5, end of surgery.
Dexmedetomidine decreases the MAP after induction to anesthesia, 5 and 20 minutes after incision (p=0.0006; 0.0009 and 0.001 respectively). The end of the surgery was with the disconnection of the infusion of Dex, what caused increase of the MAP (p>0.05). In the Dex group, in 6 patients from 30, a drop of MAP below 60 mmHg was found, which was treated with 3 mg ephedrine iv. This variation of the MAP in both groups is visible on the Chart (Figure 1).
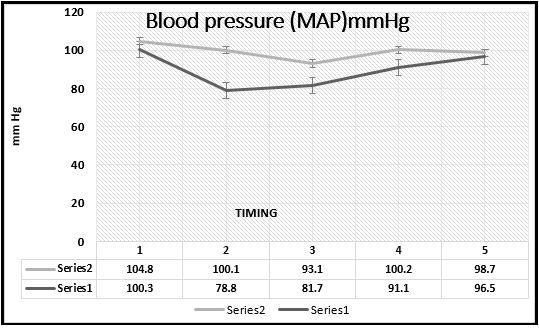
Figure 1 Mean arterial blood pressure in the study groups. Series 2, MAP of midazolam group; series 1, MAP of dexmedetomidine group.
Peroperative pulse rate in all patents sedated with Dex showed a slow drop after induction to anesthesia. It showed a statistical very significant difference from the patient receiving midazolam and was lower more than 20 times per minutes (p < 0.0000041; 0.000000036; 0.00632) (Table 6). In 10 patients the value of the pulse rate dropped ³ 50 bats/minutes (52.46±10.11). For correction of the pulse rate, they received atropine 0.05 mg.
|
Variables |
t 1 |
t 2 |
t 3 |
t 4 |
t 5 |
|
Midazolam |
82.86±7.3 |
83.7±6.3 |
78.33±6.6 |
78.33±6.5 |
77.76±5.9 |
|
Dexmedetomidine |
80.16±15.5 |
63.13±6.26 |
52.46±10.11 |
71.43±13.14 |
75.23±13.66 |
|
p |
0.141 |
4.1-18 * |
3.694-25 * |
6.32-05 * |
0.08 |
Table 6 Peroperative pulse rate/min during midazolam and dexmedetomidine sedation (M±SD)
'Procedural sedation' (PS), or 'conscious sedation', aims to help patients tolerate unpleasant painful procedures, without the possibility of unpleasant memories associated with the procedures. PS also aims to reduce the pain sensation in patients.7 Traditionally, 'analgosedation' (AS) is achieved by combining analgesics with sedatives. The use of PS/AS reduces the duration of painful and unpleasant diagnostic procedures and increases patient safety. The technique of peroperative administration of sedatives and analgesics aims to suppress a patient's consciousness to safe limits, in which breathing, oxygenation and airway patency are not affected.8 Midazolam causes minimal hemodynamic disorders but tends to lose the reflex to maintain a patent airway, causes respiratory depression and even apnea, hence its use requires experience.1 It causes anterograde amnesia. Like all benzodiazepines, its effect is due to an increased GABA inhibitory neurotransmission in the brain. It is used as a premedication at a dose of 0.07 to 0.10 mg/kg im, and for intravenous sedation 0.05 to 0.15 mg/kg iv. In this study, a dose of 0.04 mg/kg was used, which is a small dose, but with a satisfactory effect, to reduce negative properties on breathing and oxygenation, which was achieved.9,10 Patients sedated with Dex received more effective sedation compared to those sedated with midazolam The safety profile of both drugs was similar, but Dex was shown to have more advantages.11 This study confirmed that apart from mild hypotension and bradycardia at induction, which are expected and easily treated, there were no other side effects. However, the satisfaction from the anesthesia, the quality of sedation and the possibility of easy cooperation with patients, makes sedation with Dex to have an advantage over sedation with midazolam.
Based on the results obtained, it was shown that DM used for analgosedation in plastic and reconstructive surgery caused expected bradycardia and hypotension. It was also confirmed that DM used for sedation had advantages over the use of midazolam: it did not disturb breathing, provided better comfort for patients, and patients were satisfied with better perioperative analgesia.
None.
The authors declares that there are no conflicts of interest.

©2023 Kishman, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.