Journal of
eISSN: 2469 - 2786


Review Article Volume 9 Issue 1
Institute of Industrial Biotechnology, Government College University Lahore, Pakistan
Correspondence: Hamid Mukhtar, Institute of Industrial Biotechnology, Government College University, Lahore-54000, Pakistan
Received: February 14, 2021 | Published: March 26, 2021
Citation: Majeed Z, Naseer B, Shah FI, et al. Efficiency of purification techniques of human IgG – a review. J Bacteriol Mycol Open Access. 2021;9(1):15-23. DOI: 10.15406/jbmoa.2021.09.00290
This review is based on technological trends in IgG purification. Various techniques have been developed to separate antibodies from complex mixtures. Among them are the non-chromatographic and chromatographic techniques. The former consist of techniques such as precipitation, liquid to liquid extraction, and high performance tangential flow filtration. While the latter consist of anion exchange, cation exchange, hydrophobic interaction, immobilized metal affinity, mixed-mode, and bio affinity chromatography. The article also throws light on emerging trend of formulating process buffers to prevent anomalies in the antibodies being purified.
Keywords: IgG purification, chromatography, antibodies, antigen, blood, anion exchange, cation exchange
Antibodies have been controlling the technology for more than over a century but not ever more than nowadays.1 Their significant success and development leads to invention from all areas. Intensive enhancement in yield of cell culture have also raised the challenge purification methods.2 The most important IgG purification method urges the consumers to look for replacements: the bio-affinity chromatography technique using the immobilized protein-A reliably attains highest capacity capture of IgG devoid of feed condition amendments, meanwhile providing greater than 95% purity with same recovery. This is an extraordinary method but its expense surpasses its performance and it is the cause to substitute it. In 2007 a review entitled as “The Future of Antibody Purification” discuss the purification techniques with the ability to amend then-current industrial procedures as disrupting.3 The industry still requires the developments in technology in order to cope up with the growing financial burdens, even if they are disruptive The industrial progression itself is the cause of the inducement for invention in its past: the appearance of bio-similar. Upon exposure to the foreign antigens, the B cells produce the antibodies also known as the immunoglobulins which are glycoproteins in nature. After their production, these antibodies either kill the antigen or spot them exclusion, e.g. opsonization or the complement activation. The IgG class is the most abundant antibody in blood (it makes almost 80% of total immunoglobulins count in blood). IgG is mainly occurs in blood plasma and in the tissue fluids. This class of antibodies is responsible for the significant roles like (i) toxin neutralization (ii) complement activation (iii) opsonization. This is the only antibody that is capable of passing the placenta and hence providing the natural immunity to newborns at birth time.4 The immunoglobulins are very useful in terms of diagnostic purposes e.g. as a diagnostic reagent in in-vitro analysis and the conjugation of the secondary antibodies are also utilized in the recognition of several diseases in various techniques e.g. HIV (ELISA), blood typing and western blotting and also in a clinical medicine. Moreover, the antibodies can also be used for passive immunization for treating the immune-compromised patients.5 As immunoglobulins exhibits numerous significant applications, so the first part of this review includes the conventional and non-chromatographic techniques.
Many considered protein a based chromatography as a productivity blockage regardless of its efficiency.6 Various substitutes are under active study, which includes the techniques of precipitation, extraction through aqueous two-phase and the ultrafiltration techniques utilizing charged membranes.7 The crystallization techniques have been described in some reviews,8 but is not discussed here because of its no capability for the antibodies. Different chromatographic techniques are also discussed here. Kelly et al haves claimed that the chromatographic technique can complete the requirements of the industry in future. Ongoing developments support his point of view. So these methods are described in this review article under the headings of SMB (simulated moving beds) fluidized beds, and design of stationary phase for fixed bed chromatography media. Current improvements in CEC (cation exchange chromatography), HIC (hydrophobic interaction chromatography), AEC (anion exchange chromatography), SEC (size exclusion chromatography), IMAC (immobilized metal affinity chromatography), and bio-affinity chromatography are also discussed here. Safety during the purification is also discussed an evolving trend: improvements in the fractionation method, in order to avoid the hindrance in the antibody population. There are four significant methods that have the potential to ensure the safety during purification: prevention of aggregate formation; maximum removal of the contaminants through the exclusion of the Ab contaminant complexes; recovery of naïve Ab from aggregates; and the re-establishment of correct disulfide bonding. Apart from their instant applied value, these developments provide information between the interactions of Ab, purification materials and the contaminants that in turn could lead towards the progress of more efficient techniques.
Cohn’s fractionation (cold ethanol fractionation) is an old technique to separate IgG from human plasma. It is done in five fractions mainly. After separating plasma proteins, fraction II contains IgG. Conditions of this technique are following (Figure 1): This technique result in significant yield loss. Use of chromatographic techniques for purification of fraction II could avoid this loss. Modern methodology of purification illustrates better elution as depicted below (Figure 2):
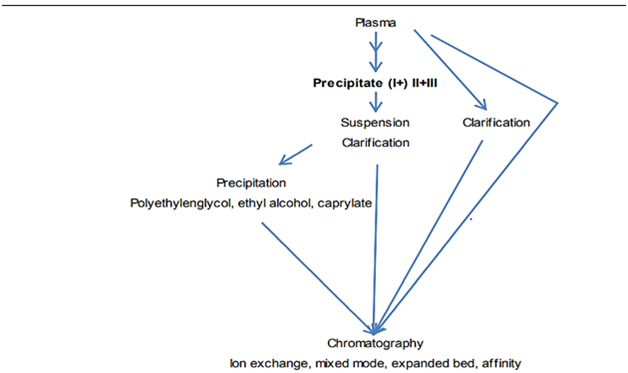
Figure 2 Modern separation technique for human IgG.46
Precipitation is considered a significant method because it concentrates the product with enhanced volume reduction and it also aids the attendant during the steps of purification.3 Due to its dependence upon the costly centrifugation it is not used in the recent procedures. Supernatant removal through the membrane filtration releases the burden for PEG (polyethylene glycol)9 as well as for ammonium sulfate10 precipitation Process improvement and the lab-scale applications can be accomplished by utilizing the disposable dead-end filtration components. Industry scale procedures can be improved with tangential flow.11 IgG have been purified from human serum, bovine serum, and cell culture supernatants of mammals.12 Mostly the IgG recovery ranges from 85-93%, with about 85% purity. Ito et al.13,14 established the modified form of ammonium sulfate precipitation that incorporates the precipitation and resuspension of antibodies. A membrane which is semi-permeable is used to separate the water channel from that of the ammonium sulfate channel, creating the gradient of ammonium sulfate leading to the protein precipitation. The precipitated species get dissolved according to their solubility due to the decrease in the concentration of salt. This procedure is greatly used for the monoclonal Ab fractionation but still it is under research that how it can be used at the commercial level fractionation. Antibodies may also be precipitated along with the polymers (negatively charged.15 The co-precipitation method ensures about 80% elimination of the host cell proteins.16 The recovery in the capture phase is exactly like CEC that is about 82% but it is comparatively lower than that of the protein A that is 95%. The removal of the protein of the host cell was only 15% effective just like in CEC. Some researchers suggested that the co-precipitation could be done in a filtration manner or in a continuous centrifugation system. Apolyallylamine based precipitation including many steps in the purification procedure attain the level of purity and recovery that is similar to the protein-A based method17 and polyarginine based precipitation method.18
The co-precipitation procedures have the ability to cause some problems as demonstrated in the caprylic acid co-precipitation procedure. It is considered as a substitute of protein A based method but it is not that much effective method.19 Reagent cost per batch of IgG exceeds the average cost per batch of protein purification. Moreover, the remaining caprylate remnants get bound with the antibody causing the hindrance in the IgG movement in agarose gels,20 and blocking the haemagglutination assays.21 The Ab recovery from the precipitates of the ammonium sulfate was obtained at 40ppm.22 Apart from the toxicity of the co-precipitating reagents, it is important to determine the guarantee of purity levels that they do not lead to the formation of particles by serving as centers of nucleation. McDonald et al.16 decreased the residual levels of poly-vinylsulfonic acid to lower than 10ppm by passing the solution of through an anion exchanger. The elimination of the polymer particles that were bound to a porous particle exchanger is tough and it also changes the media presentation but it can be minimized by using a single membrane exchanger.
High performance tangential flow filtration (HPTF)
A technique was developed by23 in which electrostatic interactions between charged ultrafiltration membranes and proteins were demonstrated (100-300kDa cutoff). Solutes have the potential to be retained due to these electrostatic interactions in ion exchange chromatography, in the process ion exclusion mediates the process of antibody selectivity. The negatively charged ultrafiltration membranes rejects the positively charges monoclonal antibodies such as IgG. Though IgG antibodies are small in size to be passed through the pores of the membrane yet can easily be repelled by the membrane. This repulsion helps in their retention while the other contaminants that are weakly alkaline, neutral or weakly acidic easily pass through the pores present in the membranes. However, a challenge is being faced by the positively charged contaminants. The DNA molecule will bind to IgG at low conductivity values that are low enough to repel the monoclonal antibodies. If the DNA molecule is present in low concentration can easily be removed by HPTF, however if the concentration exceeds a limit it would reduce the charge on the membrane and hence the intensity of repulsion of the monoclonal antibodies will also be significantly reduced. If the IgG antibody binds at the surface of a cation exchanger it would greatly reduce the ion exchange capacity.24 A sample conditioning step is required in order to make the effective use of HPTF in removing the bulk amount of DNA. Another challenge that is being faced includes the variability among the antibodies. The IgG antibodies that are strongly alkaline are highly retained and repelled by the membrane and can tolerate high salt concentration and low pH and hence a great benefit is achieved that includes the passage of the contaminants to a greater degree. However, on the other hand the antibodies that are highly acidic in nature have lower retention compared to the alkaline ones.
Liquid extraction
Advancement in the Aqueous Two Phase extraction method have begun when methods alternative to affinity chromatography have been accepted.25 The principle of the technique lies in the fact that the IgG antibodies are more readily transferred from the aqueous formulations to enriched PEG phase. An introduction about the technique has been provided in a review by Azevedo et al.26 An IgG purity of 70 to 95% and recoveries greater than 95% have been achieved in a PEG citrate sodium chloride system however the final antibody concentration is averaged at 0.2mg/ml 26 Mao et al.27 optimized the pH for the removal of host protein and found 7.2 as the optimized pH. However, the removal of aggregates and the contaminants require an optimize pH of 5.5. Oelmier et al.28 play a role in the recognition of the time period and the labor required for the experimental screening conditions and developed an automated cloud point method. The process is intended to yield partition coefficients that are identical to those generated by the methods performed manually. Investigations have also been performed on the two phase polymers such as PEG and Dextran. The PEG polymer has been used for the partition of antibodies. Hydrophobic ligands were also coupled with the terminal hydroxyl groups of the PEG polymer in order to increase the partition coefficients.29 Though after using all these parameters the purity and recovery were still the same as were in the PEG Citrate systems yet the antibody concentration was merely 1mg/ml. Ligands have been used that form more direct interactions with the proteins. A metal chelator named as iminodiacetic acid was attached by Birkenmier et al.30 A dye ligand was attached by Zilgstra31 Although these steps increase the partition efficiency however an extra step was introduced that require the separation of these bound structures from the antibody. However, on the other hand residual PEG that is unmodified is beneficial in increasing the binding capacity on the cation exchanger and hydroxyapatite.32 It also aims at highlighting the potential for immediate purification and product concentration. ATP was integrated with the cell culture in which the production of cells take place at the lower phase followed by the spontaneous transfer of IgG to the PEG phase, this prevented the prolonged contact of IgG with the dead cell debris and it also plays a role in smoothening the process of protein harvesting.31 Azevedo et al.26 in their study argued with the fact that though combining ATP increases process economy and environmental impact is being lowered but the issue remains the same. ATP is an entirely different process and it requires different hardware, combining the two processes will burden the users and complicate the entire process. With reference to the variations in antibodies variations in the ATP performance have been produced but they still need to be characterized. The virus and DNA ability in reduction also need to be characterized. The process of involved in the intermediate and final purification steps also need to be characterized that will reflect the product quality. An IgG extraction system that require the reverse surfactant micelles present in a solvent system based on iso-octane was developed by George and Stuckey (50), this process has 80-90% efficiency and 30% reduction in the biological activity was observed. Also a double activity recovery was observed by adding a non-ionic detergent i.e. Brig-30S.
Aqueous two phase extraction
ATPE for plasma fractionation is a cost effective method. PEG, sodium chloride and phosphate buffer were used in ATP system. There was 83% recovery and after polishing 92% pure IgG was collected with70% recovery.33 Method for purification is showed in following flowchart (Figure 3):
In aqueous two phase extraction (ATPE) or aqueous biphasic extraction system (ABS) is capable of extracting human IgG in a single step. Ferreira et al.34 used polyethylene glycols, buffers with some adjuvants such as ionic liquids. This was compared with adjuvant free system and exceptional result data showed that percentage purity of IgG enhanced by 37% and rate of IgG stability was also higher with IL-ABS (ionic liquid- aqueous biphasic system). Phosphate buffer, PEG were used in ATPE in continuous process in three consecutive steps i.e. extraction, back extraction and washing by Rosa et al.35 to analyze the efficiency of technique on isolation of IgG from cell supernatant of Chinese hamster ovary. Feasibility of this method due to economic sustainability and scalability made it a preferable technique of human antibody (IgG) purification.
This chromatography usually involves the usage of adsorbent particles distributed in a liquid medium. One if the simple and best example is batch chromatography, but highly-engineered flow up columns are required for the industries that have the ability to maintain the particles in a uniform dispersed state whole over the equilibration, washing and sample applications. After settlement of bed, processes such as elution, cleaning, and sanitization are basically performed. One of the versions is mainly stated as expanded bed chromatography. Particles are usually generated in order to embody a slight range of densities. When the particles are distributed upwards by the flowing buffer, heavier particles stratify to the bottom of the bed and the less heavy particle towards the top.32 Complex samples can be clearly separated by use of theoretical chromatographic plates that are formed on the basis of stable stratification process. While operating with fluidized bed it is far more complex than fixed beds. This allows the selective capture of antibodies because in this procedure cells and cell debris dispersed with particles chromatograph. Many articles and reviews describe its practical application and economical importance the broad implementation is best described in first generation limitations. The force limitation is that while performing chromatography the cell debris whose density is near to density of chromatographic particles overlap with them and cause hindrance in cleaning procedure of chromatographic material.
The Other limitation is that it has more cost then packed beds and the cost for buffer preparation and media preparation are higher. Are the problem is falling of chronic frit. These limitations are cured in second generation. Fouling can be prevented by use of rotating arm at inlet because this rotation uniformly distributes the particles. Density problems can be e overcome by use of cellulose coated tungsten carbide particles. Because use of these particles increases the difference in densities of cell and particles used in chromatographic material. It also allows efficient and fast results. Dolly beads are used that maintain the fast capturing because they maintain the residence time. If we compare the polyclonal beds with protein A of fixed beds when it is not so good but their efficiency increase with Suspending the particles and polyclonal Ig G bodies of human can be purified with 93% recovery if plasma contain 80% of it. This was achieved by mixed mode legend. At first protein a was recovered and purified and it was similar to fluidized beds but time for this procedure was less than fluidized beds. Immunoglobulins G were extracted from blood and culture media by use of magnetic microsphere. The purity and recovery of protein A and immunoglobulins G are almost same (binding capacity for 30 milligrams per ml particles). Phenylalanine can be used as legend this provides an advantage to nanoparticles. If 158 nm is the size of Nano sphere, then it has surface area of 1874 meter. Due to the binding capacity of immunoglobulins G become 780 milligrams per ml. Di particles whose size is 150 mm and when they are substituted with imidazole their binding capacity becomes up to 843 milligrams per ml.24
Diffusive particle chromatography
This method is specific for the purification of antibodies. The artic rafts for this type of chromatography are recovery, capacity dependent upon slow flow and procedure for separation. Bi features are not part of chromatography but specific for this type some people think that this type chromatography should be abandoned. Deflorate can be limited and restricted because of dependency upon diffusive mass transfer. Resolution that does not depend on the floor rate can be created by turbulence in wide volume and is proportional to flow rate. Instead of all such limitations diffusion method is used because it provides maximum accessible surface for immunoglobulin G and binding capacity that is dynamic for all fixed phases.36
Perfusive micro particle chromatography
This type of chromatography is based on the media that supports intra particle flow. Because particles are diffusive so they form trance channels from these channels solute can form access to the surface area and forms connected mass transport. Due to this fractionation and binding capacity can be achieved at higher flow rates which cannot achieve with diffusive methods. But the capacity is lower than diffusive method capacity exclusively. And they are burden when eddy dispersion and turbulent shear is performed in void volume.34
Absorptive microfilament membrane methods
Because of connection between solute and mass transfer this method allows higher efficiency in starfish uptake even at higher flow rate. In this method the pressure that is provided in operating procedure is comparatively low that helps the higher flow rates and connective transfer becomes valuable. Because transfer of connective maths does not depend upon various factors such as viscosity, flow rate and size of solute so there binding capacity is also independent of these factors. The efficiency can be independent of these factors as well but it can be changed with thickness of membrane and load distribution that can be poor from high devised volume housing designs. This method is best applicable samples that has been diluted. it has an important limitation which is that it provides low binding capacity to preteens than porous particles.37
Size exclusion chromatography
Conventional SEC with HPLC (high performance liquid chromatography) is an ideal technique for IgG purification, as this has capacity to elute the immunoglobulin with higher resolution. In SEC, silica beads have been in use due to their inert nature and ability to withstand high pressures. Columns under usage should be joined so that in less time result could be observed. These parameters were established for purification and analysis of IgG.38 Combination of techniques could be more successful method for antibody isolation and purification. ATPE with chromatographic techniques such as SEC and HIC were analyzed for this purpose. ATPE (PEG and sodium citrate) concentrated the antibodies with 97% of IgG yield, then loaded on phenyl-sepharose HIC column with 99% recovery of Abs. 100% pure IgG solution obtained after SEC phase and maximum yield (90%) was observed.29 Purification of synthesized antibodies differ from that of antibodies from natural source. Size exclusion chromatography has long been used for separation of IgG from other antibody aggregates such as that containing IgM, analysis of these antibodies can also be carried out. However, chances of non-specific hydrophobic interaction of antibody with solid phase makes it unfeasible. Using different buffers of various concentration in size exclusion chromatography media offer favorable results.15 A study was performed to analyze the efficacy of surfactant aided SEC in IgG purification. As compared to conventional SEC, SASEC showed higher yield with lesser consumption of solvent. Purity of IgG not compromised made this technique quite favorable but this result based on purification of monoclonal IgG. Isolation and purification from serum cannot give such promising results, so for this purpose combination of SEC with Hydrophobic interaction chromatography techniques could be administered.39
Cation exchange chromatography
The extraordinary aptitude of freshly presented porous particle ion exchangers has invigorated attention in CEC as capture substitute to protein A. In this with a very slight increase in salt concentration IgG can then be eluted. With hybrid pH gradient IgG has easily be eluted. Higher pH of proteins encourages removal of host cell protein. The pH control has arisen as an unforeseen trial for CEC applications. Hydrogen ions which are positively charged usually abridge opposite negatively charged cation exchange ligands throughout equilibration. Sodium ions usually have an advanced affinity for the exchange groups as compared to hydrogen ions, so thus when salt is presented, for instance upon elution or loading, it usually relocates hydrogen ions to the moveable phase, thus a gradual drop in PH occur. The pH drop usually results in exceed in 2 Ph units, contingent on the exchange, the equilibration circumstances, the resist in pH, and the salt concentration presented. If at the end of sample load, salt concentration is abridged, pH spikes raised, owing to solid phase hydrogen ions binding. With weak cation exchangers, the response is more penetrating than robust ones, but many stuffs advertised as robust cation exchangers have important concentrations on their polymer background of carboxyl groups, so product tags are not reliable indicators.4
Hydrophobic interaction chromatography
HIC is the best application for aggregate removal. A lot of examples are present on membrane absorber’s and porous particle media. Both of them are used in flow-through mode. For removal of DNA and host protein contaminants, HIC is very effective, but their applications are abridged due to increase in salt concentration at which elution of antibody occur.40 This is usually not necessary because illusion with low conductivity can usually be done with glycine don't have the ability who encourage high-capacity binding do are to molar wash can be capable to jam retention of antibody that was previously loaded in conventional binding of salts like ammonium sulphate. As glycine is beta ionic and it has a pH of 4to 8 and, it actually contributes to connectivity. Glycine can also be used to modulate selectivity in flow-through application.
Anion exchange chromatography
Anion exchangers such as DEAE (diethylaminoethyl) linked with Sepharose can be used for separation of human IgG by keeping the pH below isoelectric point of most of the antibodies. Different matrices have been under operation such as DEAE-Sepharose and DEAE-cellulose. Although it poses some drawbacks such as instability of immunoglobulin at low ionic strength but highly pure (more than 90%) IgG can be obtained from this technique.41 Another study by Ilić et al.42 depicted AEC technique as promising method for IgG fractionation from human sera. Flow rate of IgG varied as 58% adsorbed on the matrix (Q Sepharose fast flow) and 42% easily passed out of the column.
Affinity chromatography
Among other chromatographic techniques, affinity chromatography is famous for its high efficiency. Protein G Sepharose for fast flow was administered by Sennikov et al. 43 to separate human IgG autoantibodies against TNF. Various cellulose affinity membranes were designed and characterized for IgG purification by Barroso et al.44 Coupling ligand with cellulose membranes altered the adsorption capability and helped to better elute human IgG. 10%wt. cellulose membranes resulted in recovery of 2mg IgG/g while 6mg IgG/g was bound. For purification of human IgG this method evidently shows promising results Protein a (bacterial surface protein of Staphylococcus aureus) resins are preferred during polyclonal human IgG fractionation. It interacts with Fc part of Ab but some subclasses of IgG didn’t show significant fractionation results.45,46 Due to low pH, protein A chromatography has been in controversial waters as it causes conformational changes in IgG structure leading to aggregate formation. Fab region folded onto Fc region and reduction of size proved essential for elution (Table 1).47 Protein A based ligand system or natural ligand (protein G) is highly costly for large scale IgG purification so there is need of alternative synthesized ligands. Apart from that, improvement of matrices is also important. Screening of synthesized ligands and their efficiency is highly commendable as they resist chemical changes as well as avoid biological contamination. To name a few synthetic ligands, there is ApA (artificial Protein A), PAM (Protein A mimetic, TG19318) and peptide H. Peptide H showed enhanced purification of rat IgG but not in case of human IgG. At high salt concentration there was improved IgG binding with ApA.36 Peptide affinity ligands are also good choice by modifying the target amino acid sequences of target proteases i.e. by replacing natural amino acids (basic, aromatic) to unnatural analogs. These protease resistant ligands significantly increased purity and yield of human IgG above 90%.48 High productivity of antibody capture by Protein chromatography was checked by Ng et al.37 with integrated experimental and modeling approach in four steps (Figure 4): Synthetic matrices (synthetic polymer bead) with metal chelates ligands were analyzed for the efficiency in purification process. PHEMA gel beads (poly (hydroxyethyl methacrylate)) with immobilized L-histidine ligand were used with metal ions such as zinc, nickel, cobalt and copper chelated on beads. This one step separation of human IgG resulted in high IgG absorption on matrix i.e. 44.8mg/g. Hexamer peptides have similar Fc binding strength with IgG as that of Protein A (Table 2).49
|
Organism |
Subclass |
Protein A binding |
|
Human |
IgG1 |
Strong binding |
|
|
IgG2 |
Strong binding |
|
|
IgG3 |
Weak/no interaction |
|
|
IgG4 |
Strong binding |
Table 1 Binding capacity of IgG with SPA (staphylococcal Protein A)
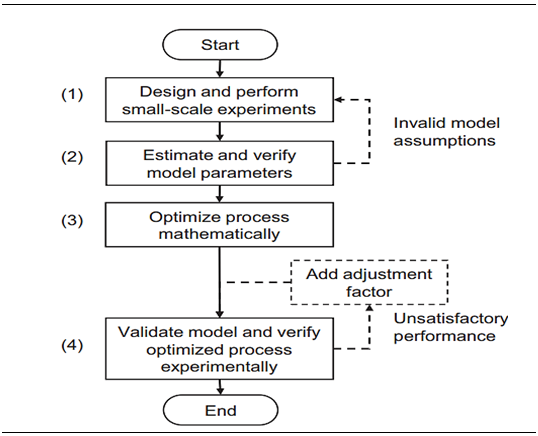
Figure 4 Integrated experimental and modeling approach for optimal chromatography design.37
|
Ligand |
Support |
IgG source |
|
Sulfamethazine |
Poly(glycidyl methacrylate) |
Human blood serum |
|
Protein A mimetic TG19318: (RTY)4K2KG |
Epoxy-activated Eupergit C30N |
Human blood serum |
|
MAbsorbent A1P/A2P |
6% Cross-link agarose |
Human blood serum |
|
2-(3-aminophenol)-6-(4-amino-1-naphthol)- 4-chloro-sym-triazine, 22/8 |
Sepharose 6B |
Human blood serum |
|
Metal ions: Ni2+, Zn2+, Co2+, Cu2+ |
Poly(hydroxyethyl methacrylate) |
Human blood serum |
Table 2 Small Ligands and their respective matrices for human IgG purification49
Among others, dye ligands have also been used in affinity chromatography. In dye ligand system, most commonly used dye is Cibracon Blue having hydrophobic and anionic characteristics. Chitosan/alginate composite as a matrix and immobilized Cibacron Blue F3GA was checked for human IgG purification after diluting serum ten times. Highly pure IgG was obtained at 53% recovery rate.50 Use of Metal-chelating ligand with 2-methacrylolyamidohistidine (MAH) co-monomer resulted in making spherical beads having diameter of 75–125mm by suspension polymerization. Poly(MMA-MAH) beads were characterized by followed by chelating Cu ions on them. For human IgG adsorption from plasma, Cu -chelated beads with a swelling ratio of 38% were used. Maximum adsorption rate was 54.3mg/g and purity percentage was 90.7%. Repeating the cycle of adsorption-desorption did not cause remarkable loss in adsorption capacity of human IgG.51
The observation of the turbidity of protein A elutes during the technique of pH neutralization was usual. And this turbid appearance indicates the formation of aggregates after getting exposed to a very low pH of the elution buffer. At low pH as the IgG antibody attains a stable conformation so the appearance of the precipitates was not obvious.52 Arginine based elution is utilized to avoid the aggregate formation during pH neutralization. Many research papers reported the information about its working.53 Arginine gets accumulated due to its interaction with the residues lessening hydrophobic interactions of IgG with protein A and also controlling the pH of elution buffer according to the requirement.54 After the neutralization of the pH of the elute, the arginine condensation at the hydrophobic portions resists the hydrophobic interactions within the IgGs. In the elutes of the arginine the aggregate level averages between 1-2% that is comparatively less than that of the other eluents.55
Removal of antibody contaminant complexes
With support of controlled glass pore and silica this suggestion was quite accurate. While in 2008 Shukla and Hinckley basically distributed a study of protein A based on polymer. They shot at in every age of greater than 90% of the contaminating cross self proteins was actually carry by protein by way of complexes with antibody that has been modelled in the cell supernatant culture. By means of secondary washers which is usually combined with when is extent of area isopropanol, they removed and dissociated majority of them. After this they come to a point that interaction was mainly hydrophobic with the potential influence by hydrogen bonding. This study was of greatest importance because it basically revealed lately and recognise phenomena with harmful potential to affect performance of purification and quality of product.55 Antibodies against human histones muesli form stable complexes with host stones that are released from cells which are dead while cell culturing is basically observed by Luhrs et al and Mechetner et Al. The antibodies are basically prevented from binding to their targeted human histones buy bound host stones the problem when it was actually proportional to the extent of cell mortality at harvest. Dissociation of foreign antigens was actually not described by conventional purification by protein and protein g affinity chromatography. This phenomenon got intricate by the statistic that histone proteins bind to DNA which is then released from cells which are dead with histones in the form of chromatin.56 Post DNA and histone proteins forced various kind of derivations on pattern potency assays thus resulting in mistake and estimates that's usually reach from 15 % to 58%. Bye using stage to method of passing supernatant that is clarified over anion exchanger and after that applying NaCl of 2 molar wash prior to eluting the columns of affinity chromatography, they were then capable to reinstate potential to the range of 90 % to 101 percent. Gangnon et al. usually determined chromatographic behaviour of antibody M- DNA complexes. DNA is usually consisting of complex maths of 24% but low levels of a lot of host cell proteins actually e deceptive on reduced SDS page. The associated DNA fragments was small when the complexes got going co-eluted with purified IGM on analytical SEC.37
Many experimental data were then observed and they showed that a combination of electrostatic interaction hydrogen bonding and metal coordination usually form association of complexes. Many of the recent studies show that basically wonder wall forces are also involved in this. Luscombe et al usually suggest that van der Val forces comprise of 65% of the bonding contacts between DNA binding domains and DNA on proteins. Rao and pohl discovered that IGT population was basically created by ferric ions with a typical late and early peaks of elution on weak cation exchanger these studies also provide abroad documentation in which they showed that contaminants and antibodies used don't coexist in biological solution lonely as independent entities, and also they warn of important consequences. Not even a single method was capable in order to complete dissociation at high columns loads. But all of the methods achieve some. Biofinity by means of second a strong wash, AEC and HA was usually attributed the capability of powerful DNA interaction to disassociate DNA from IgG. Dissociation got improved by a urea wash before elution of a AC this usually attribute to the ability of urea to weak hydrogen bond between antibody M and DNA without eliminating electrostatic interactions between exchanger and the DNA. Complexes got dissociate only by monolithic anion exchanger. Porous particle exchanger shortly fractionated them, this difference was basically greater than 10 times higher charge density on monolith urea NaCl and combined washes improves disassociation on HA.56 The combination was usually suggested to weaken hydrogen bond and electrostatic interaction between antibody and DNA, without weakening metal coordination between DNA phosphate and HA calcium. A wash of EDTA, before elution of CEC also increase complex dissociation. This study shows that complex composition stability and abundance usually define strongly with antibody characteristic and host cell level mortality at harvest. Usually more investigation is now required to show how such complexes occur and how they are able to effect performance of unification and also how they affect quality of product.57
Restoration of native Ab from aggregates
A direct loss product usually represent removal of aggregate the loss is usually compounded if fractionation need sacrificing known aggregated in antibody to confirm a lot of reduction of levels of aggregate. If it is not completely removed residue low-level aggregates usually act as a centre of nucleation for the particle development during storage of product. Disaggregation and refolding which are pressure induced usually has been applied too many proteins. Dissociation of aggregates is usually believed to be occur at high pressure by insertion of water between hydrophobic protein to protein interfaces. It also improves electrostriction of water to the level of salt bridges destruction, but the secondary structure and hydrogen bonding will remain unaffected.2000 bar hydrostatic pressure usually cause this aggregation. A pressure doubled than that is required for narrative structure and folding FC fusion protein was basically exposed to PH 3 to form aggregate of 14.5%.57 This is usually not necessary because low conductivity can usually be done with glycine don't have the ability who encourage high-capacity binding do are to molar wash can be capable to jam retention of antibody that was previously loaded in conventional binding of salts like ammonium sulphate. As glycine is beta ionic and it has a pH of 4 to 8 it actually contributes to connectivity.52
Novel approaches in IgG purification
Engineered protein A with thermosensitivity capacity can be considered as a novel approach for IgG purification. Mutant protein A binds with IgG at 5-degree C and at 40-degree C IgG is released. Under neutral conditions, IgG can be purified with Thermo-responsive protein A (TRPA) column by fluctuating the temperature. By this technique, isolated IgG is in the form of monomer. Antigen binding ability of Antibody does not decrease in this way. Therefore, this novel approach can be successfully used on industrial scale.57
Elution of IgG in series of processes has not only become critical process but economic aspect is still under debate. Without denying the importance of purified IgG having various applications it is essential to keep tract of techniques of purification. Most compromising methods of purification are HIC as it does not change the IgG conformation. Some techniques give high yield but their cost is much higher so cannot be adopted at industrial scale such as protein A chromatography. Magnetic nanoparticles bead in affinity chromatography can also be used for faster elution. With progression of technology, combination of techniques has proved vital for highly pure IgG. As protein A can be bioengineered, this novel approach has solved some big issues regarding purification.58–64
A best method is to develop a platform for antibody purification which require less time and expense. This approach is quite feasible and scientists are working on it and it is a big challenge for them because of diversity of antibodies and generation of complex antibody linked derivatives. However, the transition in antibody purification has usually started and many companies are being evaluating mixed modes as the replacements for anion exchange chromatography. Hydrophobic anion exchanger has a leading start, with a lot of start, but the enhanced H-bonding anion exchanger has taken a momentum. the development of method with the former has usually turned out to be initiative. It usually levies cooperation between the final quality of product and its recovery. The last one has conversant sense of outdated anion exchangers, with the chief alteration being their aptitude to bind acidic contaminants above a much broader range of conditions. Removal of aggregate is usually an inspirational choice of intermediate methods of purification. Hydrophobic anion exchangers and hydroxyapatite are existing because of their coextensive aptitude to lessen levels of DNA, the viruses, percolated protein A, and host proteins which are acidic, but none of them has confirmed completely acceptable. The appearance of novel diverse modes will perhaps retain this area of fluid for a long time. A best method is to develop a platform for antibody purification which require less time and expense. This approach is quite feasible and scientists are working on it and it is a big challenge for them because of diversity of antibodies and generation of complex antibody linked derivatives.
None.
The authors declare that there is no conflict of interest.

©2021 Majeed, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.