Journal of
eISSN: 2469 - 2786


Research Article Volume 5 Issue 5
1Department of Plant Protection, Agriculture and Natural Resources Research Center of Mazandaran, Iran
2Plant Protection Departments, Islamic Azad University Damghan Branch, Iran
Correspondence: Hessam Moddin Hassani, Plant Protection Departments, Islamic Azad University Damghan Branch, Iran, Tel +98(0)9124318258
Received: August 01, 2017 | Published: November 21, 2017
Citation: Barari H, Dalili SA, Hassani HM. Genetic diversity among different isolates of Sclerotinia sclerotiorum in north of Iran. J Bacteriol Mycol Open Access. 2017;5(5):387-389. DOI: 10.15406/jbmoa.2017.05.00151
Sclerotinia sclerotiorum (Lib.) de Bary is a cosmopolitan, homothallic fungus, and is the most important causal agent of stem rot diseases in field crops of Iran. During 2011-2012, a total of 52 isolates of the fungus were collected from infected rapeseed plants (Brassica napus cultivar Hiola 401) from several fields of Northern Iran. The genetic diversity of S. sclerotiorum populations were assessed using Simple Sequence Repeat fingerprinting (SSR). By using five SSR primers, 27 haploid groups with 14 alleles polymorphism were detected. A high level of genetic diversity 52% was observed between isolates. The results showed that there were possibly high rates of out crossing, as well as, evolutionary potential within 52 isolates obtained from different geographical locations. The variability found within closely related isolates demonstrates the effectiveness of SSR marker in identifying genetic diversity. This is the first report on genetical diversity in S. sclerotiorum populations in Mazandaran province in northern Iran.
Keywords: microsatellites, sclerotinia sclerotiorum, genetic diversity
Sclerotinia sclerotiorum (Lib.) de Bray an ascomycetous fungus, causing white mould disease on more than 400 agricultural and wild plant species has a worldwide geographical distribution.1,2 S. sclerotiorum is a homothallic fungus which disperses by either airborne ascospores, or by soil-borne sclerotia. The sclerotia are the resting propagules that germinate to produce either hyphae or apothecia.3-6 Sclerotinia rot is the most destructive disease of canola, especially in favourable climatic conditions,in the northern flats of the Caspian Sea.7 In Iran, the evaluation of several cultivars and genotypes of rapeseed against Sclerotinia rot has shown different levels of tolerance.8
Control strategies must target a population, instead of an individual, if they are to be effective.9 In plant-pathogen interactions, The development of new pathogenic races, and the breakdown of host resistance are the limiting factors in resistance deployment against plant diseases, The pathogen’s life history characteristics and evolutionary potential are also major factors for pathogen is ability overcoming in the host resistance.10,11,14 Therefore, major efforts should be focused not only in understanding the genetic structure of the fungal populations, but also to determine how populations will evolve in response to different control strategies.12
Two independent mechanisms, Mycelial Compatibility Group (MCG) and DNA fingerprinting, are known to differentiate S. sclerotiorum populations. DNA fingerprinting technique can also be used to distinguish closely related fungal isolates. Southern hybridisation of restriction digested whole genomic DNA to a cloned probe containing a 4.5 kb repeated dispersed element of nuclear DNA from S. sclerotiorum has been used previously.13-15 Microsatellites,16 also known as simple sequence repeats (SSRs), Or short tandem repeats (STRs), are the smallest class of simple repetitive DNA sequences,12,17 that are widely dispersed and evenly distributed in the genome of eukaryotes, has also been used to study intraspecific genetic variability within populations.10,18 The aim of this present research was to investigate the genetic structure within the oilseed rape population of S. sclerotiorum in the Mazandaran province of Iran based on molecular markers.
Pathogen isolates
S. sclerotiorum isolates were collected from 11 rapeseed fields in Mazandaran province of northern Iran during 2011-2012 crop season. Sclerotia from infected plants were removed and a single sclerotium was selected as an isolate. Sclerotia were surface sterilized for 1 min. in 70% ethanol or 2 min. In 2.5% sodium hypochlorite, rinsed in sterilized-distilled water, plated on potato dextrose agar medium (PDA), and incubated at 22 ̊C for two days. Each isolate was purified by transferring the single hyphal tip onto the fresh medium, and the generated sclerotia were stored at -20 ̊C until use.19,20
DNA extraction
Isolates were grown on PDA and incubated at 21- 24 ̊C in darkness for 5 days. Mycelia were harvested by vacuum filtration, lyophilized, and stored at -20 ̊C. Fifteen to 20 mg of dried mycelia were powdered, and transferred to 1.5 Eppendorf tubes for DNA extraction following the method of Liu et al.21 with the slight modifications. The DNA pellets were dissolved in 30 μl of 1X TE (10 mM Tris-HCl, 1 mM EDTA), and stored at 5 ̊C.21
Three sets of microsatellite primers including CATA25, CA9, and TACA10 were used in this study.18 Gels were stained with ethidium bromide, visualized under UV light, and digitally documented with the gel documentation UVP-V system. The gel was run at 90 W for 90 min.22 All polymorphic alleles were identified from each microsatellite primer combination, and bands representing alleles were scored as present (1) or absent (0). Nei’s genetic distance matrix23 was prepared, and bootstrap analysis with 2000 replications, was performed to generate a dendrogram of unweighted pair-group mean analysis24 using the TREECON 1.3b program.25
Polymorphism information content (PIC)
The polymorphism information content (PIC) determining the frequency of alleles polymorphism in the gene locus of the population, was calculated as follows:
PIC= 1-Σn i=1Pi2
Where pi was the relative frequency of it alleles.
Genetic diversity among the Sclerotinia sclerotiorum isolates
In 52 isolates the microsatellite primers exhibited 14 clear polymorphic alleles. The number of polymorphic alleles per locus ranged from 3 to 6 (Table 1). The genetic relationships among the isolates was determined by using a separate matrix to the data from the 27 polymorphic alleles, and the 52 isolates was determined by using a separate matrix to the data from the 27 polymorphic alleles, and the 52 isolates were then clustered (Figure 1).
Locus (Accessions Motifs No.) |
Repeat |
Primer Sequence (5 |
PIC |
Allele Number |
Previous Reports |
Other Species |
12-2 AF377906 |
(CA)9 |
CGATAATTTCCCCTCACTTGC GGAAGTCCTGATATCGTTGAGG |
0.388 |
3 |
4a |
No |
106-4 AF377921 |
(CATA)25 |
TGCATCTCGATGCTTGAATC |
0.781 |
6 |
10a |
Yes |
55-4 AF377918 |
(TACA)10 |
GTTTTCGGTTGTGTGCTGG |
0.611 |
5 |
7a |
Yes |
Table 1 Microsatellite and polymorphic loci, and polymorphism information content (PCR) for S. sclerotiorum
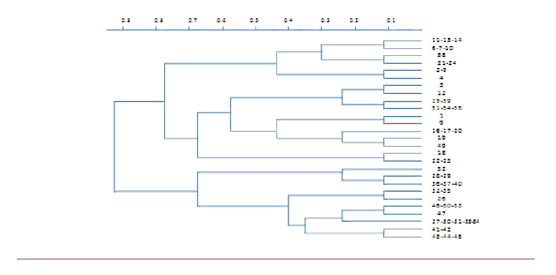
Figure 1 Unweighted pair-group analysis dendrogram of genetic distance among the 65 fungal isolates based on jaccard’s Coefficients.
The polymorphism information content (PIC) for each locus ranged from0.388 for (CA) 9 to 0.781 for (CATA) 25 (Table 1). Therefore, the Locus (CATA) 25 contained the most identified alleles and the highest PIC, although a high level of diversity was observed between the clusters, some isolates within clusters were identical for all the microsatellite markers. For instance, isolates 41 and 42 had similar alleles for all the microsatellite markers. Overall, 27 distinct isolates were identified among the 52 isolates.
In the present study, it was discovered that microsatellite markers were very efficient in identifying genetic variation among the isolates. Three of these marker sets identified polymorphism among the Iranian isolates. When our findings were compared with the previous work,20 Four new alleles, one at (TACA) 10 and (CA) 9, and three at (CATA) 25, were discovered.18,20
Results of our present study showing presence of 27(S) different clones (haplotypes) among the 52(N) isolates strongly suggests that a high degree of genetic variability (S/N%=52%) exists in the S. sclerotiorum population in the mazandaran province of northern Iran. From Australia Sexton & Howlett26 also reported presence of genotypic diversity, ranging from 36-80%, amongst S. sclerotiorum isolates collected from four oilseed rape fields. From New Zealand, Carpenter et al.27 have also reported shared microsatellite haplotypes amongst S. sclerotiorum isolates collected from different fields.
Shared haplotypes among populations indicate either considerable gene flow (exchange of haplotypes among populations) and the populations represent the same founder population, shared haplotypes among populations could also be the result of sexual reproduction, i.e. inbreeding or out-crossing among similar genotypes. The resultant ascospores would be genetically similar and appear clonal. Because ascospores can be dispersed over long distances,15 it is not surprising to find shared clonal genotypes among several regions. Shared microsatellite haplotypes among populations have also been reported from Australia and North America.20,26,27 Diverse crops are cultivated, and where environmental conditions favour sexual recombination within S. sclerotiorum. Oilseed rape is a new crop to this area and the high level of polymorphism may reflect the movement of S. sclerotiorum ascospores onto rapeseed crop from several wild host plants.28
None.
The author declares no conflict of interest.

©2017 Barari, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.