Journal of
eISSN: 2469 - 2786


Research Article Volume 3 Issue 3
Department of Botany, University of the Punjab, Pakistan
Correspondence: Ambreen Ahmed, Department of Botany, University of the Punjab, Lahore, Pakistan, Tel 00923334595101
Received: November 07, 2016 | Published: December 13, 2016
Citation: Aslam T, Ahmed A. Growth improvement studies in brassica rapa. J Bacteriol Mycol Open Access. 2016;3(3):246-249. DOI: 10.15406/jbmoa.2016.03.00062
PGPR are the plant growth promoting rhizobacteria that enhance plant growth by various mechanisms. Auxin production ability of microbes affects plant-microbe interaction and enhances their plant growth promotion ability. So, harmful effects of fertilizers can be avoided by the application of PGPR. In the current work, bacterial strains were isolated, characterized and screened for their auxin production. Bacterial strains with high auxin production ability were used to monitor their effects on various growth and biochemical parameters of Brassica rapa.
Keywords: PGPR, Rhizobacteria, brassica rapa, phytohormones, phosphorus solublization
Today the global population has reached to 7.3 billion and is expected to cross 10 billion in 2050. This shocking rise in population is generating countless stresses on the available agricultural areas for food, fiber, fuel and raw materials. Application of better plant varieties and technological involvement seems to be very important to fulfill the needs of rising population. The demand for increasing crop productivity by embracing more concerted agronomic applications and excessive and repeated use of fertilizers has deteriorated soil health. However, beneficial rhizobacteria are thought to improve plant growth.1
The bacteria that grow within the vicinity of the rhizosphere are termed as rhizobacteria. Plant growth is enhanced by the application of plant growth promoting rhizobacteria (PGPR).2,3 The selection of rhizosphere proficient microbes with plant growth improving characteristics is necessary for increasing crop yield.4 PGPR utilize a number of direct and indirect methods for increasing plant growth. Mechanisms such as nitrogen fixation, phosphorus solublization, siderophore production, plant growth regulators, organic acids and different enzymes including ACC-deaminase, chitinase and glucanase help to promote growth in plants.5 Efficacy of PGPR inoculations regarding plant growth promotion and crop production relies upon their ability to thrive and reproduce in soil and is affected by several abiotic and biotic factors.6 Presence of various substances around the roots including amino acids, sugars and organic acids are responsible for increased colonization ability of bacteria.7 Auxins produced by bacteria act as signaling molecules for association between bacteria to synchronize their activities. There is increasing confirmation that auxins indirectly regulate numerous plant processes by modulating the expression of many genes.8 Natural as well as artificial auxins stimulate or inhibit root growth, root hair production and root cluster formation depending on its concentration.3 The application of PGPR is rapidly rising in agriculture because it presents an effective practice to reduce the utilization of chemical fertilizers and affiliated agrochemicals.9 Brassica rapa (Turnip) is used in the present study to analyze the impact of rhizobacteria. It is a biennial crop containing swollen tuberous taproot with green, hairy or bristly leaves. Its root as well as green leaves are edible. It is also grown for feeding livestock during winter. Turnip is a starch vegetable and is considered as a rich source of insoluble fiber, antioxidants, folic acid, manganese, niacin, potassium, riboflavin and vitamin A, B, E and especially vitamin C and contains low calories. It can be used to cure a number of diseases like scurvy, colon cancer, breast tumors, rheumatism, cataracts and muscular degeneration as well as bronchial disorders (http://www.diethealthclub.com/health-food/turnip-health-benefits.html).
Isolation and characterization of bacterial strains
Auxin producing PGPR were isolated from the rhizosphere of various plants. After purification, the isolates were characterized. Morphological characterization included colony and cell morphology. Physiological characterization of isolated strains was done for optimizing conditions for their growth such as temperature, pH. Growth curve of bacterial strains was also studied by inoculating bacteria for various periods of time i.e., 24, 48, 72 and 96 hours of incubation and recording their optical density at 600nm.
Plant-microbe interaction
Certified seeds of Brassica rapa var. Golden Ball were provided by Punjab Seed Corporation, Lahore, Pakistan. Petri plates (120mm) were washed, autoclaved and oven dried after placing two Whatman paper no. 1 in each plate. Seeds of Brassica rapa var. Golden Ball were sterilized with 0.1% HgCl2 and were treated with the bacterial cultures adjusted to the same optical density i.e., 105 to 106 CFU. For control treatment, seeds were treated with autoclaved distilled water for the same time period. Treated seeds were uniformly spread on the filter paper contained in sterilized and labeled petriplates. All the petriplates were kept in dark for three days at 25˚C. After three days, germination of the seeds was noted and seedlings were transferred to the labeled pots containing 7.37 kg sieved soil. Four seedlings were transferred in each pot and three replicates for each treatment were used for the study. The pots were kept in the wire house under natural conditions. The plants were harvested at maturity and various growth and biochemical parameters of the treated and non-treated plants like germination percentage, shoot length, root length, number of leaves, weight of turnip, diameter of turnip, soluble protein content and auxin content were recorded.
Statistical analysis
The data obtained were statistically analyzed using the software SPSS v. 16.
In the present study, various cultivated plants were collected from the premises of Lahore i.e., rice (Oryza sativa), wheat (Triticum aestivum), cabbage (Brassica oleraceae), sugar beet (Beta vulgaris) and potato (Solanum tuberosum). Seventy seven bacterial strains were isolated from the rhizosphere of the selected plants and were screened for their auxin production ability. Out of 77 isolates, only nine bacterial strains showed high auxin production potential and these were used for further research work(Table 1). These bacterial strains were characterized morphologically and physiologically. Colonies of all bacterial strains (P1iv, S4ii, S4iv, T10ai, T8bi, T2aii, T4b, W6i and W6ii) were round, entire and opaque except S4ii. Elevation and colony shape also vary widely. Cells of the bacterial isolate T8bi were non-motile whereas all other strains have motile cells. Three bacterial strains i.e., P1iv, T4b and W6i were gram-negative whereas all other (S4ii, S4iv, T10ai, T8bi, T2aii and W6ii) were gram-positive. Colony size of the isolates varied from 0.2-0.5mm (Table 2). The strains P1iv and T4b showed optimum growth at 37˚C whereas all other strains (S4ii, S4iv, T10ai, T8bi, T2aii, W6i and W6ii) exhibited optimum growth at 25˚C. Maximum growth of most of the isolates (P1iv, S4iv, T8bi, T4b, W6i and W6ii) was observed in media adjusted at pH 5. The isolate T10ai was neutrophilic as it exhibited maximum growth at pH 7. The bacterial strains P1iv, S4iv, T10ai, T8bi, T2aii, T4b, W6i and W6ii showed maximum growth after 24 hours of incubation while the strains S4ii and T10ai showed maximum growth after 48 hours of incubation.
Isolates |
|||||||||
P1iv |
S4ii |
S4iv |
T10ai |
T8bi |
T2aii |
T4b |
W6i |
W6ii |
|
Colony Color |
Mustard Yellow |
Yellow |
Mustard yellow |
Off-white |
Light yellow |
Off-white |
Off-white |
Off-white |
Off-white |
Colony Shape |
Round |
Oval |
Round |
Round |
Round |
Round |
Round |
Round |
Round |
Colony Margins |
Entire |
Dentate |
Entire |
Entire |
Entire |
Entire |
Entire |
Entire |
Entire |
Colony Elevation |
Concave |
Concave |
Convex |
Convex |
Concave |
Convex |
Convex |
Concave |
Convex |
Clarity |
Opaque |
Transparent |
Opaque |
Opaque |
Opaque |
Opaque |
Opaque |
Opaque |
Opaque |
Cell Shape |
Cocci |
Cocci |
Rods |
Cocci |
Rods |
Cocci |
Rods |
Rods |
Cocci |
Gram Staining |
G –ve |
G +ve |
G +ve |
G +ve |
G +ve |
G +ve |
G -ve |
G -ve |
G +ve |
Motility |
Motile |
Motile |
Motile |
Motile |
Non-motile |
Motile |
Motile |
Motile |
Motile |
Spore Production |
+ |
+ |
- |
- |
+ |
+ |
+ |
- |
+ |
Colony Size (mm) |
0.3-0.4 |
0.3-0.4 |
0.2-0.3 |
0.3-0.4 |
0.3-0.4 |
0.4-0.5 |
0.2-0.3 |
0.4-0.5 |
0.2-0.3 |
Table 2 Colony and cell morphology of isolated bacterial strains
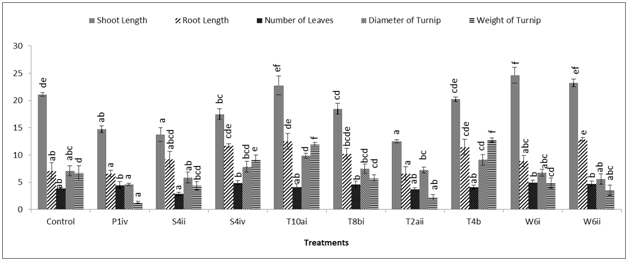
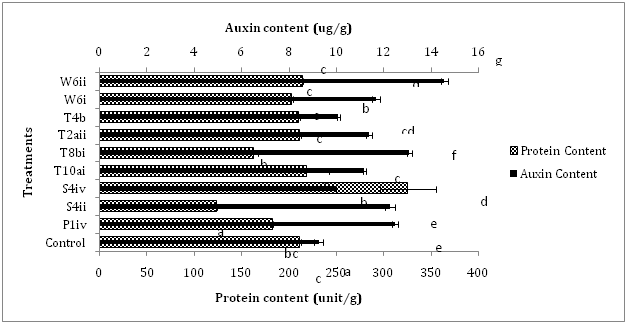
The plant-microbe interaction experiment was conducted to evaluate the effect of auxin-producing bacteria on the growth and biochemical parameters of Brassica rapa. Increase in germination percentage was found in all the inoculated treatments except T10ai as compared to control. Treatment of plants with the bacterial strain T2aii showed maximum increment of 170% in seed germination over control. The bacterial isolates T8bi and T4b showed enhancement of 150% in the germination percentage of inoculated plants whereas P1iv and W6i caused 125% increase in germination of seeds in comparison to control treatment (Figure 1). Approximately 137.5 and 62.5% increase in germination percentage was recorded by inoculation of seeds with the bacterial isolates W6ii and S4ii, respectively. Shoot length of inoculated plants was enhanced by treatment with the bacterial strains W6i, W6ii and T10ai i.e., 16.6, 10.0 and 7.7%, respectively, over control. Reductions up to 40.8, 35.0, 30.1, 17.1, 12.4 and 4.1% were recorded in shoot length of plants inoculated with the bacterial strains T2aii, S4ii, P1iv, S4iv, T8bi and T4b, respectively, when compared with the control. The root length of Brassica rapa was increased by inoculation with the bacterial strains W6ii, T10ai, S4iv, T4b, T8bi, S4ii and W6i i.e., 80.7, 75.4, 64.9, 61.4, 43.9, 28.9 and 24.6%, respectively over control treatments. However, inoculation with the bacterial strains P1iv and T2aii reduced (7.0%) the root length as compared to control (Figure 1). The isolates W6i, S4iv, W6ii, T8bi, P1iv, T10ai and T4b increased the number of leaves up to 29.0, 25.8, 22.6, 19.4, 16.1, 6.4 and 6.4%, respectively over non-inoculated control but the number of leaves of plants treated with the bacterial strains S4ii and T2aii was reduced up to 25.8 and 3.2% respectively, in comparison to the control. The diameter of bulbous tap root showed enhancements up to 38.6, 28.0, 10.5, 5.2 and 1.8% by treatment with the isolates T10ai, T4b, S4iv, T8bi and T2aii, respectively as compared to the control. However, treatment with the bacterial strains P1iv, W6ii, S4ii, and W6i caused decrease in the diameter of Brassica rapa up to 35.0, 21.0, 17.5 and 5.3%, respectively over control. Likewise, plants treated with the bacterial strains T4b, T10ai and S4iv caused enhancement in the weight of turnip root upto 90.9, 78.2 and 38.5%, respectively over control. Some bacterial strains such as P1iv, T2aii, W6ii, S4ii, W6i and T8bi also showed reduction in the weight of turnip root i.e., 79.8, 66.1, 47.4, 34.3, 28.0 and 14.6%, respectively when compared to control (Figure 1). Inoculation of plants with the isolate T2aii has shown no effect on the soluble protein content. The protein content was enhanced by treatment of plants with the isolates S4iv, T10ai and W6i i.e., 53.6, 3.5 and 1.4% respectively, over control. Treatment with the isolates S4ii, T8bi, P1iv, W6i and T4b reduced the protein content up to 41.5, 22.8, 13.7, 4.2 and 0.5%, respectively, as compared to the control. Inoculation with all the bacterial isolates increased the auxin content of plants as compared to the control. Increment of 57.2, 41.4, 34.9, 32.4, 26.3, 23.0, 20.9, 9.0 and 7.9% in auxin content was observed over control by inoculation with the isolates W6ii, T8bi, P1iv, S4ii, W6i, T2aii, T10ai, T4b and S4iv, respectively over non-inoculated control treatment (Figure 2).
Nine bacterial isolates with auxin production potential were isolated and evaluated for their capacity to increase the growth of plants. Out of nine selected isolates, three were gram-negative and other six were gram-positive. Six bacterial strains (T8bi, P1iv, S4ii, T2aii, T4b and W6ii) were spore-formers and remaining were non-spore formers (Table 2). Cells of the bacterial strains S4iv, T8bi, T4b and W6i were rods whereas remaining strains were having cells which were cocci in shape. Generally, temperature of 25˚C, pH of media adjusted to 5 and 24 hours incubation period were the physiological conditions that were found suitable for optimum growth of most of the isolates.
The germination percentage was highest for the plants inoculated with the isolate T2aii i.e., 170% over control. All the treated plants showed significant enhancement in percentage germination. Other workers like Lenin et al.10 have reported increment in percentage germination of Catharanthus roseus due to enhancement in secretion of phytohormones in the spermosphere. Inoculation of Brassica rapa seeds with the isolates T10ai, W6i and W6ii enhanced the shoot length, root length and number of leaves when compared with the control treatments. Inoculation with the isolates W6i, W6ii and T10ai caused significant enhancement of 16.6, 10.0 and 7.7%, respectively in the shoot length over control. Similarly, increment of 80.7, 75.4 and 24.6% was recorded in the root length by treatment of seeds with the isolates W6ii, T10ai and W6i, respectively in comparison to non-inoculated plants. Similar results showing increment in the shoot and root lengths of groundnut (Arachis hypogaea L.) were obtained by Mathivanan et al.11 Three isolates also increased the number of leaves of plants after inoculation. The enhancements of 29, 22.6 and 6.4% were shown by the isolates W6i, W6ii and T10ai, respectively in the number of leaves as compared to control treatment. Besides these, bacterial strains S4iv, T8bi and P1iv also enhanced (25.8, 19.4 and 16.1%, respectively) number of leaves over control (Figure 1). Other workers have also reported maximum increment in number of leaves per shoot in biostimulant inoculations as compared to the control.12 The bulbous taproot portion of turnip stores excess amount of food thus increased diameter of turnip means more food available or high nutritious contents. Inoculation of plants by the bacterial isolates T4b, T10ai and S4iv increased the diameter as well as weight of turnip roots. The increment of 38.9, 28 and 10.5% was shown by treatment with the bacterial strains T10ai, T4b and S4iv, respectively as compared to control. Likewise, treatment with the isolates T4b, T10ai and S4iv showed enhancements upto 90.9, 78.2 and 38.4%, respectively over non-treated plants. The protein content of plants inoculated with the isolate S4iv caused significant enhancement (53.6%) whereas inoculation with the bacterial isolates T10ai and W6ii also showed enhancements i.e., 3.5 and 1.4%, respectively over control. However, plants inoculated with bacterial strains S4iv, T10ai and W6ii increased auxin content i.e., 7.9, 20.9 and 57.2%, respectively as compared to non-inoculated control (Figure 2). Thus the auxin-producing bacterial isolates not only improved growth parameters but resulted in significant enhancements in biochemical parameters of Brassica rapa as well which manifested the overall improvement in the plant development affecting the internal biology of treated plants in a beneficial manner.
Present study suggested that application of plant growth promoting bacteria has significant effect on the growth as well as biochemical parameters of Brassica rapa. Overall, all strains improved plant growth. But inoculation of plants with the bacterial isolates T10ai and T4b enhanced the diameter and weight of turnip which actually enhances the food storage capacity and nutrient content of turnip. Thus, the application of bacterial strains to improve plant growth is very effective and is an indication to minimize the use of chemical fertilizers which are the root cause of many diseases in mankind. In addition, these fertilizers are responsible for gradual increase in the soil as well as water pollution in the environment. So, PGPR strains can be used as bio fertilizers in agriculture systems.
University of the Punjab is acknowledged for providing funds for the present research work.
The author declares no conflict of interest.

©2016 Aslam, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.