Journal of
eISSN: 2373-4396


Research Article Volume 17 Issue 1
1Faculty of Biology and Medicine, Lausanne University Hospital, Switzerland
2Service of Nephrology and Hypertension, Department of Medicine, Lausanne University Hospital, Switzerland
3Service of Nephrology and Hypertension, Inselspital, Switzerland
Correspondence: Dr Michel Burnier, Emeritus Professor, Derrey le Motty 8 1806 Saint-Légier, Switzerland
Received: February 19, 2024 | Published: February 29, 2024
Citation: Burnier M, Maillard M, Vogt B, et al. Effect of angiotensin II receptor blockade combined with a thiazide diuretic on plasma aldosterone escape in healthy subjects. J Cardiol Curr Res. 2024;17(1):16-21. DOI: 10.15406/jccr.2024.17.00599
Objective: The chronic administration of ARBs is associated with an incomplete ALDO suppression suggesting either a partial blockade of adrenal angiotensin AT1 receptors or a breakthrough phenomenon. We have investigated the adrenal response to exogenous Ang II in healthy subjects receiving an ARB alone or in association with a thiazide diuretic.
Methods: Thirty subjects participated in this randomised, controlled, single-blind study. The effect of a 1 h infusion of Ang II (3 ng/kg/min) on plasma ALDO levels were investigated in each volunteer before and after a 1-week single-blind administration of either irbesartan 300 mg alone or in association with hydrochlorothiazide (HCTZ) 12.5 or 25 mg. After one week washout period, volunteers were switched to olmesartan 20mg/HCTZ 25 mg or valsartan 160/HCTZ 25 mg or losartan 100/HCTZ 25mg for another week.
Results: Compared to pre-treatment values and to irbesartan alone, trough plasma ALDO values were higher in subjects receiving Irb300/12.5, and were significantly increased with Irb300/25 (to 4.9±1.8 and 7.5±1.2 ng/dl respectively, p<0.05). In participants receiving Olm20/25, Vals160/25 and Los100/25, trough ALDO were also significantly elevated. Before any treatment, exogenous Ang II increased plasma ALDO acutely from 3.6±0.7 to 13.2±1.3 ng/dl. With Irb300, the ALDO response to Ang II was blunted by 50-60% independently of the presence of HCTZ (p<0.05). The ALDO response to Ang II was also significantly blunted by about 50-60% with Olm20/25 and Vals160/25, but not with Los100/25.
Conclusion: These results demonstrate that recommended doses of most ARBs only partially block the adrenal response to exogenous Ang II when associated with a thiazide diuretic. The ARB-thiazide association accentuates the aldosterone escape thus further justifying the use of mineralocorticoid antagonists in some clinical circumstances such as resistant hypertension or hypertension with severe end organ damage.
Keywords: Aldosterone escape, angiotensin II, angiotensin receptor blockers, thiazide diuretics, potassium
RAS, renin-angiotensin system; ACEIs, angiotensin converting enzyme inhibitors; ARBs, angiotensin II receptor blockers; ALDO, aldosterone; GFR, glomerular filtration rate; HCTZ, hydrochlorothiazide; SBP, systolic blood pressure; BP, blood pressure; HR, heart rate
Blockade of the renin-angiotensin system (RAS), traditionally with angiotensin converting enzyme inhibitors (ACEIs) or angiotensin II (Ang II) receptor blockers (ARBs) represents an efficient and well recognized therapeutic strategy in the management of essential hypertension1 and congestive heart failure.2,3 At the currently recommended doses, blockade of Ang II synthesis or activity is also an efficient and well-tolerated treatment for limiting the progression of type 2 diabetic nephropathy as well as some non-diabetic nephropathies.4
Both ACEIs and ARBs have been shown to lower plasma aldosterone (ALDO) levels acutely.5,6 However, the repeated administration of RAS blocking agents is frequently associated with an incomplete ALDO suppression and in some cases with a paradoxical increase in ALDO levels, suggesting ALDO breakthrough.7,8 The two large-scale trials performed with mineralocorticoid receptor antagonists in patients with congestive heart failure have highlighted the potential impact of high aldosterone levels on morbidity and mortality.9,10 In addition, the recent demonstration of the benefits of the non-steroidal mineralocorticoid antagonist finerenone in patients with a diabetic nephropathy has further emphasized the clinical interest of combining a RAS blocker with an aldosterone antagonist.11,12 The non- epithelial profibrotic and pro-inflammatory effects of ALDO, particularly within the cardiovascular, have been well studied.13 Thus, ALDO breakthrough could contribute to cardiovascular and renal damages in hypertension particularly when associated with a high salt intake.14,15 ALDO escape has also been associated with a faster decline in glomerular filtration rate (GFR) in patients with diabetic or non-diabetic nephropathies.16,17 At last, an increase in ALDO to renin ratio has been associated with an increased incidence of either ischemic or hemorrhagic strokes.18
In clinical practice ACEIs and ARBs are often combined with a thiazide diuretic in order to make blood pressure (BP) more Ang II-dependent, and hence to lower BP more effectively.1 However, thiazide diuretics are known to stimulate the RAS by salt depletion, as well as loss of extra-cellular fluid volume, and the resulting increase in Ang II is a potent stimulus for ALDO release. We have demonstrated previously that ARBs prescribed at their current maximal recommended doses do not block renal tubular AT1 receptors as effectively as vascular receptors in healthy subjects.19 Nevertheless, to the authors’ knowledge, the degree of adrenal tissue AT1 receptor blockade provided by ARBs has never been investigated in humans during chronic RAS blockade and even less so in subjects receiving a RAS blocker and a thiazide diuretic. This raises the question of whether ARBs used at their recommended doses, initially determined to efficiently block AT1 vascular receptors, are sufficient to match the additional thiazide stimulation of RAS and to provide a full adrenal AT1 receptor blockade. In case of RAS activation, an incomplete blockade of AT1 receptors could actually contribute to the ALDO breakthrough phenomenon.
In order to address this question, the current study compared the ALDO response to exogenous angiotensin II in subjects receiving irbesartan alone or in association with hydrochlorothiazide (HCTZ) 12.5 or 25 mg and in the same subjects receiving one of three other ARBs combined with a thiazide in healthy subjects. Under each treatment the degree of adrenal AT1 receptor blockade was assessed before and after one week drug administration changes in serum potassium were also measured.
Participants
Thirty-four male Caucasian volunteers were recruited for this study. After explaining the nature and purpose of the study, informed written consent was obtained from each participant. All volunteers underwent a complete physical examination and a detailed medical history. Routine laboratory tests were performed at screening. The protocol was approved by the Ethical Committee of the Canton de Vaud, Lausanne, Switzerland.
Two volunteers were excluded for co-existing medical conditions, and thus thirty-two healthy, normotensive, male volunteers were enrolled in this study. One participant was excluded just after the pre-treatment assessment day and one did not complete the second period of treatment, both for unrelated health reasons. Hence, data of the first period were deleted as well. Finally, a participant randomized to receive irbesartan/HCTZ 12.5 mg was unable to sufficiently empty his bladder under study conditions and thus urinary parameters were not included.
Study design
The protocol of this study had been published previously.19 In brief, in this single-blind, randomized cross-over study, volunteers were allocated to receive two treatments. Each treatment lasted seven days, and there was a washout period of at least one week between the two treatment periods.
During the first period of treatment of 1 week, participants were randomly assigned to one of three treatment arms: irbesartan 300 mg once daily (o.d) (n=10), irbesartan 300 mg + HCTZ 12.5 mg (o.d) (n=9-10) and irbesartan 300 mg + HCTZ 25 mg (o.d) (n=10). For the second period of treatment, individuals on irbesartan 300 mg were blindly switched to losartan 100 mg+ HCTZ 25 mg (o.d), those on irbesartan 300 mg + HCTZ 12.5 mg to olmesartan 20 mg + HCTZ 25 mg (o.d), and those on irbesartan 300 mg + HCTZ 25 mg to valsartan 160 mg + HCTZ 25 mg (o.d).
Biochemical and clinical assessments of participants were carried out on three assessment days: just before the first treatment period (referred to as “pretreatment”), and 24 hours after the last dose of each period of treatment. Participants were allowed free sodium and water intake throughout the study. 24-hour urinary sodium excretion (U24NaV) was measured by urine collection during the 24-hours before each assessment day.
Measurements during the assessment day were taken during three consecutive one-hour blocks. In the first hour, vascular, renal and adrenal parameters (referred to as “trough” values) were measured. Throughout the second hour, exogenous Ang II was infused. ALDO response was measured immediately after the Ang II infusion, and again after the third hour, referred to as recovery.
Before each assessment day, volunteers underwent an overnight fast. Once comfortably placed in the supine position, a venous catheter was inserted into an antecubital vein to collect blood. A second venous catheter was inserted into an antecubital vein of the other arm to perfuse Ang II. The following trough parameters were measured: blood pressure (BP), urinary sodium and potassium excretion, as well as serum creatinine, potassium and ALDO levels. Plasma and urinary creatinine were measured using the Jaffe method.
Ang II (Clinalfa, Switzerland) was infused at a rate of 3 ng/kg/min because this dose is known to increase plasma ALDO levels by about 10-12 ng/dl19 and systolic blood pressure (SBP) by about 10-15 mmHg in healthy individuals. ALDO was measured in unextracted human plasma samples by a commercially available direct radioimmunoassay (ALDO Coat-A-Count, DPC, Los Angeles, CA, USA). Blood pressure (BP), heart rate (HR), body weight and safety assessments were performed regularly during the study periods. All potential clinical and biological side effects were recorded.
Statistical analysis
Results are expressed as mean±standard error of the mean, unless otherwise specified. Using SPSS version 15.0.1 (IBM, Chicago, IL), statistical analysis was performed by repeated- measures analysis of variance, followed by a paired Student’s t-test when appropriate, with an alpha criterion of 0.05. Trough parameters under treatment were compared to trough parameters at pretreatment and ALDO responses to exogenous Ang II under treatment were compared with pretreatment responses. Due to the small number of individuals in each group, between group analyses were not performed.
Participants were aged 25±3 (mean±SEM) years and weighed 78±8 kg. Pre-treatment BP was 119±1/ 66±1 mmHg, and heart rate 57±1 beats/min. Haemoglobin was 152±5 g/l, serum creatinine was 89±7 mmol/l and mean 24-h urinary sodium excretion was 117±11 mmol/day. There was no difference between randomized groups at baseline. All drugs were well tolerated and no significant adverse event was recorded during the study. Measurements following the recovery hour did not provide additional information, and therefore will not be presented. No significant change in BP was observed in subjects receiving irbesartan and HCTZ whereas small but significant changes in systolic BP (-4 to -7 mmHg) were observed with valsartan, losartan and olmesartan when associated with HCTZ.
Effect of adding HCTZ on plasma aldosterone levels and aldosterone response to exogenous Ang II
Baseline trough plasma ALDO values, and the changes in response to a one-hour infusion of exogenous Ang II 3 ng/Kg/min, tested before and after a one-week treatment with irbesartan 300 mg alone or in combination with HCTZ 12.5 or 25 mg are presented in Figure 1a. In comparison to pre-treatment values, trough plasma ALDO had not changed after one week of irbesartan alone. By contrast, trough plasma ALDO levels tended to increase when HCTZ 12.5 mg was added to irbesartan (from 3.6±0.7 to 4.9±1.8 ng/dl, p=ns) and were significantly higher with the addition of HCTZ 25 mg (7.5±1.2 ng/dl, P=0.05). In the pre- treatment period, the injection of exogenous Ang II increased plasma ALDO from 3.6±0.7 to 13.2±1.3 ng/dl (P<0.001 vs. pre-Ang II). Under irbesartan, the response to exogenous Ang II was significantly blunted by about 50-60% independently of adding the thiazide diuretic with an increase of plasma aldosterone levels to 8.2±0.6 for irbesartan, 9.0±1.1 for irbesartan/ HCTZ 12.5 and 12.1±2.1 ng/dl for irbesartan/ HCTZ 25 mg. Of note, with HCTZ 25 mg, the post-Ang II ALDO level was not significantly different when compared to post-Ang II ALDO value at pre-treatment. Figure 1b shows the changes in plasma aldosterone levels after one week of irbesartan, olmesartan, valsartan and losartan all combined with 25 mg HCTZ. Trough plasma ALDO levels were significantly higher than in the pre-treament phase with all ARBs associated with HCTZ 25 mg (to 7.8±1.7 ng/dL (P=0.01) with olmesartan, to 8.1±1.5 ng/dL (P=0.001) with valsartan and to 6.7±2.25 ng/dl (P=0.04) with losartan. As observed with irbesartan, the ALDO response to exogenous Ang II was significantly blunted by about 50-60% with all ARBs combined with HCTZ 25 mg, except with losartan/HCTZ. Indeed, losartan 100/25 mg did not significantly block ALDO response 24h after dosing.
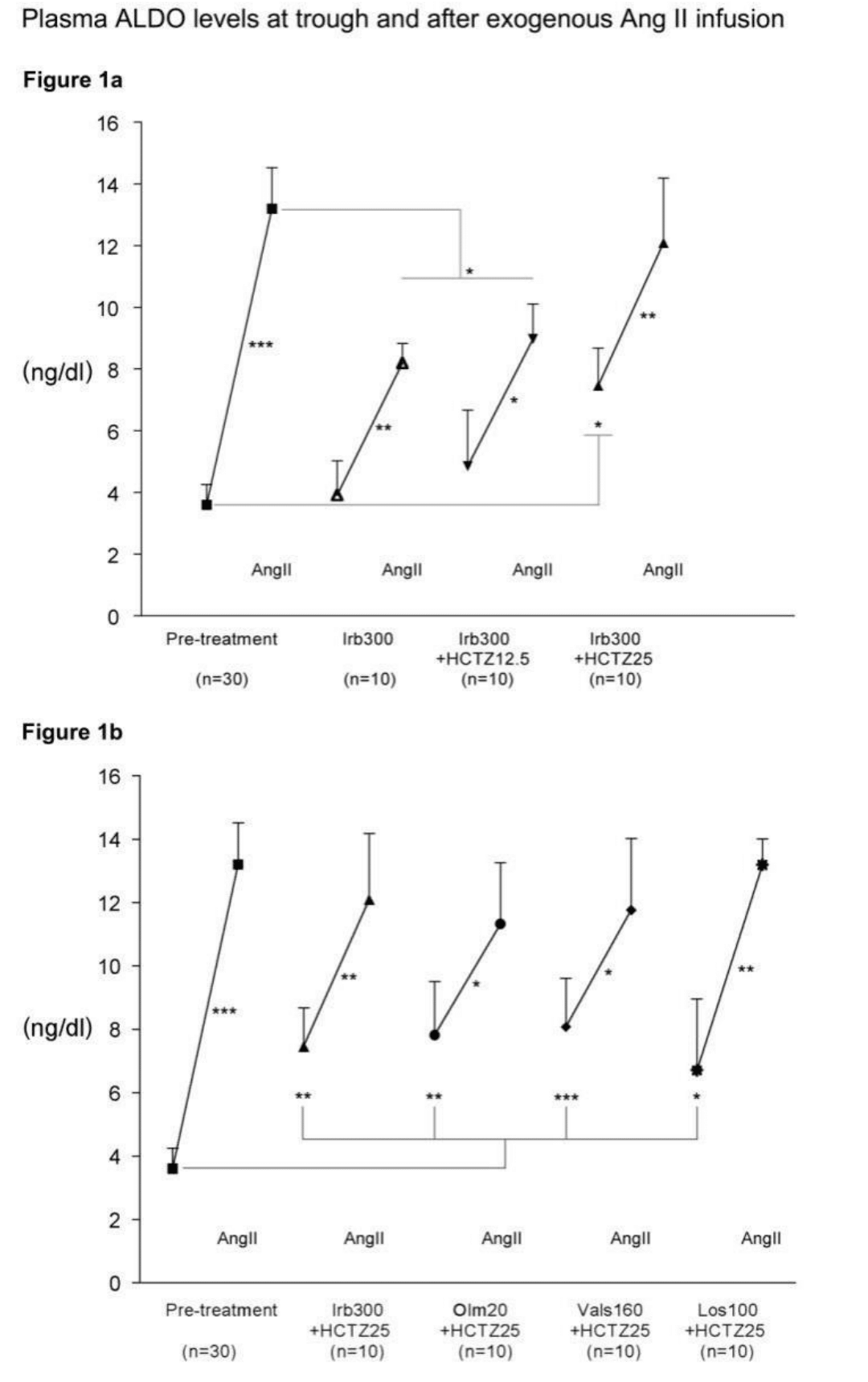
Figure 1 1a: Comparative effects of a one-week treatment of either irbesartan 300 mg alone or combined with hydrochlorothiazide 12.5 mg or 25 mg on the trough plasma aldosterone and change in plasma aldosterone in response to a 1-h infusion of angiotensin II (3 ng/kg/min), compared to pre-treatment response.
1b: Comparative effects of a one-week treatment of either irbesartan 300 mg, olmesartan 20 mg, valsartan 160 mg or losartan 100 mg all combined with hydrochlorothiazide 25 mg on the trough plasma aldosterone and change in plasma aldosterone in response to exogenous angiotensin II, compared to pre-treatment response, for the same participants. n=30 for the pre-treatment values and n=10 for each treatment group. *P < 0.05, **P < 0.01, ***P < 0.001. Ang II, angiotensin II; ALDO, aldosterone; HCTZ, hydrochlorothiazide; Irb, irbesartan; Olm, olmesartan; Vals, valsartan; Los, losartan.
Effect of adding HCTZ on serum potassium levels
The combination of ARBs and HCTZ 25 mg on serum potassium measured before and after a one-week treatment seemed to depend on the pharmacological profile of the ARB as shown in Figure 2. With long-acting blockers (irbesartan and olmesartan) no significant change in serum potassium was observed with the combination whatever the dose of HCTZ. In contrast, with the shorter acting ARBs (valsartan and losartan) a significant trend towards hypokalemia was observed. Thus, upon addition of HCTZ 25 mg, serum potassium decreased significantly to 3.8±0.1 with valsartan (P=0.02) and to 3.7±0.1 mmol/l with losartan (P=0.04).

Figure 2 Comparative effects of a one-week treatment of either irbesartan 300 mg, olmesartan 20 mg, valsartan 160 mg or losartan 100 mg all combined with hydrochlorothiazide 25 mg on serum potassium. n=30 for the pre-treatment values and n=10 for each treatment group. *P < 0.05: before Ang II under treatment vs. before Ang II at pre-treatment. Ang II, angiotensin II; HCTZ, hydrochlorothiazide; Irb, irbesartan; Olm, olmesartan; Vals, valsartan; Los, losartan.
The results of the present study demonstrate that: 1) the adrenal response to exogenous angiotensin II is only partially blocked by ARBs; 2) adding HCTZ 25 mg to an ARB potentiates the aldosterone breakthrough thus increasing baseline plasma aldosterone levels and this whatever the duration of action of the ARB, and 3) the diuretic-induced increase in plasma aldosterone is accompanied with significant changes in serum potassium, which appear to be more pronounced with short acting ARBs.
The first interesting observation made in this study is that blockade of adrenal AT1 receptors is not complete when healthy subjects receive the maximal recommended dose of several ARBs for one week in contrast with what has been observed acutely.20 In a previous analysis of the same dataset, we have shown that the blood pressure response to exogenous Ang II was completely abolished with the same doses of ARBs but the renal hemodynamic and tubular responses were only partially blunted suggesting also an incomplete blockade of renal AT1 receptors.19 In the present analysis, we demonstrate a similar phenomenon in the adrenal gland with only about 50% blockade of AT1 receptors with the long-acting ARBs. This observation may contribute to explain the well-known ALDO breakthrough that has been described with the repeated administration of ACE inhibitors as well as ARBs.21 Thus, the increase in circulating Ang II consecutive to the blockade of AT1 receptors by ARBs may stimulate the remaining unblocked AT1 receptors in the adrenals and thereby contribute to the long-term increase in plasma aldosterone levels.
To our knowledge, nobody has investigated the impact of associating HCTZ to an ARB on the aldosterone breakthrough phenomenon. The second interesting finding is that the coadministration of a diuretic enhances the aldosterone escape phenomenon of ARBs. Indeed, this is illustrated by the trough aldosterone levels measured after one week of irbesartan alone or in combination with 12.5 or 25 mg HCTZ. Our results clearly show an increase in trough plasma aldosterone levels already with irbesartan alone when compared to pre-treatment values but aldosterone levels are even higher when increasing doses of HCTZ are added. The mechanisms whereby HCTZ can stimulate aldosterone are well recognized and are linked to salt depletion and transient reductions in plasma volume and activation of the RAS.22 Our finding however further suggests that adrenal angiotensin II receptors are not completely antagonized and that adrenal glands are always able to respond to changes in sodium and volume balance by producing aldosterone. One of the consequence of the aldosterone escape in subjects receiving thiazide diuretics, may be an attenuation of the renal tubular response to the thiazide diuretic, sodium being reabsorbed more distally in aldosterone-sensitive tubular segments.
In our study, several ARBs with different duration of action were investigated in association with 25 mg HCTZ. This enabled to investigate the interaction between the intensity and duration of RAS blockade, the use of HCTZ and the breakthrough phenomenon. In our hands, all ARBs blunted the adrenal response to exogenous Ang II by about 50% at trough except losartan, which has a shorter duration of action resulting in only a 30% blockade of AT1 receptors 24h after dosing. However, at trough, plasma aldosterone levels were comparable in all ARB groups receiving HCTZ, though slightly lower in the losartan group. This suggests that the escape phenomenon observed when ARBs are combined with a thiazide diuretic, is a physiological renal response to avoid excessive salt losses, which may actually result in volume expansion.
One puzzling question is why are adrenal AT1 receptors partially escaping blockade? AT1 receptors are present in very high density in adrenal glomerulosa cells.23 Consequently maximal steroidogenic response may occur at Ang II concentrations saturating only partially the available receptors. The higher number of AT1 receptors in glomerulosa cells may result in a left-shifted dose-response curve for Ang II biological effects. Moreover, experimental studies have shown that Ang II concentrations are high in adrenal glands and that the regulation of the renin-angiotensin system in the adrenals might differ from that of the circulatory or renal systems.24 The local production of angiotensin II might explain why the renal and adrenal response to angiotensin receptor blockade differs from that observed in the vasculature. The partial blockade of adrenal AT1 receptors by ARBs in case of RAS activation by HCTZ may be due to high adrenal AT1 receptors density together with high local Ang II concentrations during AT1 receptor blockade and administration of the diuretic.
Several other hypotheses have been proposed to explain the ALDO breakthrough. With the use of ACE inhibitors, it has been suggested that non-ACE tissue Ang II synthesis could stimulate ALDO.25 With ARBs, corticotrophin, changes in serum potassium secondary to RAS blockade, as well as V1a vasopressin receptor activation have been proposed to explain the breakthrough phenomenon. Furthermore, with prolonged renin-angiotensin-aldosterone (RAAS) overstimulation, hypertrophy and hyperplasia of adrenal glomerulosa cells, as well as ALDO synthase induction have been reported, which may also play a role in ALDO breakthrough. Other factors have been shown to contribute to ALDO synthesis such as endothelin-1 (ET-1),26 serotonin, T-type calcium channels and bone morphogenetic protein-6 (BMP-6).27 In addition, AT2 receptors could be implicated in ALDO breakthrough by sensitizing the adrenal cortex to Ang II. Our results cannot exclude a participation of AT2 receptors in ALDO breakthrough under study conditions. However, the AT2-hypothesis seems to be less likely. Indeed, in case of RAS activation, we would expect a left-shift in the Ang II biological curve effects and an increased sensitivity of the adrenal gland to Ang II. Thus, we would expect an increase in ALDO secretion during Ang II infusion, which was not observed in our subjects
The long-term use of HCTZ is associated with dose-related side effects, particularly hypokalemia, one of the most frequent pharmacologically-induced electrolytic disturbances. In contrast, blocking the RAS with ARBs tends to increase serum potassium by lowering plasma aldosterone. In 1999, Kochar et al.28 have reported that hypokaliemia induced by HCTZ was blunted in a dose-related manner when the diuretic was combined with irbesartan. Thus ARBs have the potential to offset some of the side-effects of thiazide diuretics. A similar observation was made in our healthy subjects after one week of irbesartan administration. However, our data provide an additional information i.e the impact of the duration of action of the ARB on the interaction with HCTZ. Indeed, our results show that ARBs with a shorter half-life are associated with lower serum potassium at trough thus confirming that long-acting blockers such as irbesartan and olmesartan are more effective in blunting the HCTZ-induced hypokalaemia. Thus, the use of long-acting blockers may be more effective in avoiding HCTZ-induced chronic hypokalaemia, which has been associated with cardio-metabolic complications such as ventricular arrhythmias, muscle weakness or chronic metabolic alkalosis.
The observations made in the present study using healthy subjects may have important implications for hypertensive patients treated with ARBs and diuretics. The incomplete blockade of adrenal AT1 receptors and the increased aldosterone breakthrough when a diuretic is added to an ARB may have some clinical consequences. Indeed, excessive aldosterone production has been associated with the induction of oxidative stress, endothelial dysfunction, inflammation and tissue fibrosis. Thus, in patients with hypertension and diabetes mellitus, the aldosterone escape has been observed in 22% of patients treated with candesartan or valsartan and was associated with a greater urinary albumin excretion, which could be blunted with the addition of an aldosterone antagonist.29 Today, several studies have shown that adding a MR antagonist to classical RAS blocking treatment could be beneficial to further lower BP in resistant hypertension, to increase cardiac protection after myocardial infarction, to decrease mortality in heart failure and to reduce morbidity and mortality in patients with diabetic or non-diabetic chronic kidney diseases.1-4,30-32 Our findings are in accordance with these observations and may constitute an additional rationale for the use of mineralocorticoid receptor antagonists together with ACEIs or ARBs in selected clinical conditions.
None.
None of the authors have a conflict of interest with this analysis.

©2024 Burnier, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.