Journal of
eISSN: 2373-633X


Case Report Volume 14 Issue 5
1Assistant Surgeon of the Surgical Clinic "A", Hospital de Clínicas Universidad de la República, Uruguay
2Surgical Resident of the Surgical Clinic "A", Hospital de Clínicas Universidad de la República, Uruguay
3Associate Professor of the Surgical Clinic "A", Hospital de Clínicas Universidad de la República, Uruguay
4Professor, Surgical Clinic "A", Hospital de Clínicas Universidad de la República, Uruguay
Correspondence: Ulises Parada, Assistant Surgeon of the Surgical Clinic "A", Hospital de Clínicas Universidad de la República, Montevideo, Uruguay, Tel 099313877
Received: November 10, 2023 | Published: November 20, 2023
Citation: Parada U, Girardi F, Fernández L, et al. Gastric GIST: a typical laparoscopic resection guided by high endoscopy. J Cancer Prev Curr Res. 2023;14(5):117-120. DOI: 10.15406/jcpcr.2023.14.00533
Gastrointestinal stromal tumors (GIST) are the most common mesenchymal neoplasms of the gastrointestinal tract. They can originate in any sector of the gastrointestinal tract and represent 0.1 to 3% of all gastrointestinal tumors. Their most frequent topography is gastric (60%). In reference to the surgical treatment of gastric GIST tumors, these depend on several factors to be taken into account; the extension of the lesion (tumor stage), the size of the lesion and the topography. The fundic location close to the esophageal-gastric junction increases the risks of generating an eventual narrowing. The surgical treatment of tumors in this topography is a therapeutic challenge because it implies a mixed technique; combining laparoscopic surgery with endoscopy. The aim of the following article is to present the combined laparoscopic and endoscopically guided treatment of an atypical gastric resection for a GIST tumor. The combination of laparoscopic surgery and endoscopy are complementary techniques that provide safety and can be a valid therapeutic option in selected cases, especially in those with tumor location close to the esophageal-gastric junction.
Keywords: gastric GIST, diagnosis, localization, surgical treatment, endoscopic treatment
Gastrointestinal stromal tumors (GIST) are the most common mesenchymal neoplasms of the digestive tract.1 They originate from interstitial cells of Cajal or even from their precursors found in the myenteric plexus.2,3 They can originate in any sector of the gastrointestinal tract and represent 0.1 to 3% of all gastrointestinal tumors. Its most frequent topography is the gastric (60%) followed by the small intestine (30%).4
It typically expresses CD117 and has mutations in the KIT gene or in platelet-derived growth factor receptor A alpha (PDGFRA).5,6 These mutations lead to activation of these cellular pathways (responsible for regulating cell proliferation, apoptosis, differentiation, adhesion, and motility under normal conditions) resulting in spontaneous proliferation and uncontrolled cell growth.
The diagnosis of GIST relies heavily on immunohistochemistry techniques. The inmmunophenotype is positive for the markers CD 34, CD 117 or DOG1. The CD 34 marker is present in 70–80% of cases, however, it is less sensitive and specific than CD117 and DOG1 because it can also be observed in leiomyomas. Most GISTs are CD 117+. DOG1 is expressed in GIST even when CD117 is negative.
The clinical presentation of gastric lesions varies widely from small, asymptomatic tumors discovered by chance, to large aggressive tumors with the capacity to spread. Symptomatology is not specific and depends mainly on the size, topography and growth pattern of the lesion. In relation to the topography of the lesions, the gastric fundus is the most frequent, close to 25%, followed by the body and antrum, although in other series the first place is occupied by the gastric body.7,8
With regard to diagnosis, GIST are part of the lesions known as subepithelial lesions (LSE), originating in the deep layer of the digestive tract. This eventuality makes diagnosis difficult since in general upper endoscopy and biopsies of the overlying mucosa do not provide a definitive histological diagnosis. Endoscopic ultrasonography (EUS) is the technique of choice to characterize LSE. EUS-guided fine needle puncture is considered a mini-invasive and safe method for biopsy acquisition, especially in lesions suspected of GIST.9
The treatment of GISTs depends largely on the extent of the disease. Roughly speaking tumors smaller than 2 cm can be followed EUS, resection surgery with margin, is the primary treatment of localized lesion larger than 2 cm. In locally advanced lesions, where complete resection is not possible or requires major mutilation, preoperative treatment with tyrosine kinase inhibitors can be initiated. In case of unresectable or metastatic disease, imatinib1,10,11 is recommended as first-line treatment.
The aim of the following article is to present the combined laparoscopic and endoscopically guided treatment of an atypical gastric resection for a GIST tumor.
Female patient aged 76 years. Personal history of arterial hypertension, type 2 diabetes, left nephrectomy (renal oncocytoma). The current history includes a history of 6 months of evolution due to atypical dyspepsia, epigastric pain, early fullness and intermittent melena in the last months. Hemoglobin of 10.4 mg/dl.
Upper gastrointestinal endoscopy: at body level there is a 4 cm round protruding lesion, with healthy mucosa. Suspected GIST. Multiple biopsies are taken on biopsy. Pathological anatomy: chronic gastritis in activity with intestinal metaplasia. Presence of H. Pylori. In an interdisciplinary meeting of the Esophageal Gastric Unit of the Hospital de Clínicas, it was decided to perform EUS and biopsy. Echo endoscopy: hypoechogenic, rounded lesion with well-defined borders, heterogeneous center as usually seen in necrosis or hemorrhage, originating in the muscularis propria layer. Ultrasound-guided fine needle biopsy. Subepithelial lesion probably GIST. Pathological anatomy: sections with histopathological elements compatible with gastric GIST. CD117 (-); CD 34 (-); DOG 1 (+).
The patient was staged with computed tomography of the thorax, abdomen and pelvis. At the gastric level there is a polypoid lesion that protrudes towards the lumen at the level of the fundus of 40 mm. It presents heterogeneous enhancement with contrast medium. No adenomegaly or hepatic or pulmonary metastases are observed. No free peritoneal fluid is observed (Figure 1).

Figure 1 Abdominal staging computed tomography. Polypoid lesion is shown in gastric lumen. It is marked with a black arrow.
It was decided to perform a laparoscopic atypical gastrectomy guided by high intraoperative endoscopy. Atypical gastric resection is performed with mechanical suture with a violet charge next to the esophageal-gastric junction.
Post-operative care in a moderate care room, with controlled pain and tolerating water 24 hours post-operative. Discharged 6 days after surgery tolerating soft diet and without pain. She denies dysphagia. Pathological anatomy: lesion of 45x40x25 mm distant 10 mm from the surgical margin. Microscopy GIST tumor, with number of mitoses 5 in 10 per field of high magnification (400x), the margin is negative without lymphovascular or perineural invasion. TNM: pT2, Nx.
A control tomography was performed 3 months after surgery without elements of tumor remnant at gastric level. No focal hepatic lesions of note, nor lesions suggestive of bone substitution. No adenomegaly, no free fluid.
In reference to the surgical treatment of gastric GIST tumors, these depend on several factors to be taken into account; the extension of the lesion (tumor stage), the size of the lesion and the topography.
In tumors larger than 2 cm that can be resected, surgery is the treatment of choice. However, it is not the only aspect to consider when choosing the surgical tactic. The topography of the lesion is an element to take into account since it can condition to achieve free resection margins a major resection such as subtotal or total gastrectomy if necessary. This can be the case of lesions close to the esophageal-gastric junction, antrum or lesser curvature.
GIST surgery has distinctive characteristics that influence the surgical technique. First of all, complete resection of the lesion with negative margins must be achieved (R0 surgery).12 Another aspect to be taken into account is to avoid opening the tumor by avoiding injury to the pseudocapsule. This fact may even condition the approach; for lesions smaller than 5 cm the laparoscopic approach seems to be an effective and safe technique. Lesions above this size increase the risk of tumor opening with consequent peritoneal seeding and risk of recurrence.13 Finally, these lesions rarely present lymph node involvement and therefore do not require lymphadenectomy in addition to tumor resection.
Tactical therapeutic aspects.
Atypical resection without lymphadenectomy has been shown to be beneficial for this type of lesions due to the characteristics seen above. The fundic location close to the esophageal-gastric junction (EGJ) increases the risks of generating an eventual narrowing. Surgical treatment of tumors in this topography is a therapeutic challenge because it involves a mixed technique; combining laparoscopic surgery with endoscopy.
What benefits it offers:
Perhaps as a negative aspect, gastric insufflation may hinder gastric mobilization, however, aspiration of the air contents quickly empties the gastric chamber.
Surgical technique and recommendations
The patient is placed in the dorsal decubitus position with the thoracoabdominal region shifted, which in the opinion of the authoring group facilitates surgery of the supra mesocolic floor. The trocars will be placed supra umbilical 10mm for optics. The rest under direct vision and following the triangulation principles. In both hypochondrium trocars 12 mm these will be used by the surgeon we recommend to place them in the centers of the legs. The size of the trocars is to allow the passage of mechanical suture. At the epigastric level a 5 mm trocar is used to assist in lifting the liver (Figure 2).

Figure 2 Marking of sites for trocar placement. Supra umbilical 10 mm for optics, in both hypochondrium 12 mm for surgeon and epigastrium 5 mm for assistance.
Once the trocars are placed, we examine the entire peritoneal cavity in search of lesions that have gone unnoticed by imaging studies. We proceed to the instrumental palpation of the gastric lesion which is identified in the anterior cardia (Figure 3).
Once identified we place a polyglactin 910 stitch as shown in figure 4. This allows traction of the lesion which greatly facilitates the passage of the mechanical suture. At this point we perform upper endoscopy to observe the lesion and endoluminal topography (Figure 5).

Figure 4 Stitch over the tumor, which allows traction and mobilization of the lesion to facilitate the passage of the mechanical suture.

Figure 5 Endoscopic view of the lesion confirming the topography on the anterior aspect of the cardia. The arrow points to the subepithelial tumorization and stars the visualization of the optic lumen.
On some occasions it may be necessary to mobilize the greater or lesser curvature in order to reduce visceral resection, this maneuver should be selective and not systematic since it increases the risks and surgical time. The same comment deserves the liberation of the fixation means of the UEG which are essential for the esophagocardiofundic statics. They will be released as long as they allow a more economical gastric resection.
Once the stitch has been made, traction is applied towards the zenith in order to elevate the tumor and perform the passage with mechanical suture underneath. Before performing the shot, we corroborate with endoscopy the existence of a sufficient diameter that does not generate an orificial syndrome as well as the inclusion of the lesion in the resection. After this, we perform the suture, for which the author group prefers the violet load (triple row of hooks) articulated to reduce and economize the gastric resection. The advantage lies in not performing a manual reinforcement suture and reducing operative times (Figure 6, 7).
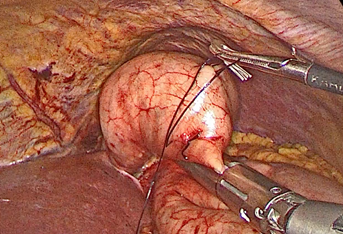
Figure 6 Traction towards the zenith of the tumor. Suture thread secured with metal clip is shown. Passage of hinged mechanical suture with violet load below the lesion.

Figure 7 Gastric section. Several shots are required, the articulated suture allows better splicing and at the same time more economical resection.
At the end of the section we perform endoscopy to ensure a good passage and a correct lumen without narrowing. It also allows us to corroborate hemostasis. The gastric chamber is insufflated and warm saline is placed in the abdominal cavity in order to perform an airtightness test (Figure 8, 9). This last test is based on the fact that most of the time more than one staple shot is required and splicing these shots increases the risk of leakage.

Figure 8 Final endoscopy after resection. It shows that there are no macroscopic tumor remains, no active bleeding and a wide and good endoluminal passage.

Figure 9 Suture tightness test. Physiological saline is instilled and the gastric chamber is insufflated with an endoscope, if no bubbles are observed the suture is airtight.
The specimen must be removed in a bag to avoid wall contact with the tumor lesion. The extraction is performed by the minimum necessary enlargement of the hypochondrium port. Subsequently performing the parietal closure.
The piece is sent for evaluation by extemporaneous pathological anatomy to corroborate the presence of the tumor and free margins. We do not leave drains.
The combination of laparoscopic surgery and endoscopy are complementary techniques that provide safety and can be a valid therapeutic option in selected cases, especially in those with tumor location close to the esophageal-gastric junction.
None.
Authors declare that there is no conflict of interest.

©2023 Parada, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.