Journal of
eISSN: 2373-633X


Review Article Volume 12 Issue 4
1DDS, National Cancer Institute, Brazil
2Ph.D. in Oral Pathology, National Cancer Institute, Brazil
3Ph.D. in Oncology, National Cancer Institute, Brazil
Correspondence: Héliton Spíndola Antunes, Rua André Cavalcanti, 37-3ª andar-Anexo-Sala 11, Bairro de Fátima-CEP: 20231-050. Rio de Janeiro, Rio de Janeiro, Brazil, Tel +55 21 32076597, Fax +55 2132076566
Received: July 08, 2021 | Published: July 19, 2021
Citation: Alves LDB, Menezes ACS, Costa AMD, et al. Strategies for the dentist management of cancer patients: narrative literature review. J Cancer Prev Curr Res. 2021;12(4):111-121. DOI: 10.15406/jcpcr.2021.12.00463
Aim: To present the dental procedures necessary for the prevention, diagnosis, and treatment of oral toxicities related to cancer treatment.
Material and methods: A narrative literature review was carried out regarding the topics of interest. Articles in Portuguese and English, without the restriction of the year of publication and full text available in the databases Pubmed, Scielo, Cochrane, and Google Scholar were investigated.
Results: Through this search, 100 articles were selected, consisting of 9 systematic reviews, 3 systematic reviews with meta-analysis, 48 literature reviews, 3 randomized clinical trials, 4 prospective cohorts, 12 retrospective cohorts, 5 cross-sectional studies, 2 case-control studies, 5 case reports, 7 expert consensus panels, 1 letter to the editor and 1 qualitative study, published between 1976 and 2020. Dental procedures were classified according to the therapeutic modality (surgery and radiotherapy for head and neck cancer, chemotherapy, hematopoietic stem cell transplantation, and use of antiresorptive medications) and the time of treatment (prior, during, or after each one).
Conclusions: The consulted articles were unanimous regarding the need for dental care for cancer patients in the pre, trans, and post-treatment, highlighting the importance of inserting specialized dental surgeons in the oncology patient care team; thus, this professional should act in the prevention, early diagnosis, and treatment of oral toxicities, to minimize their consequences and improve patients' quality of life.
Keywords: dentistry, medical oncology, patient care team, radiotherapy, chemotherapy, hematopoietic stem cell transplantation, antiresorptive medications
According to data released by the International Agency for Research on Cancer (IARC) in 2020, cancer will be the second leading cause of premature deaths in most countries including Brazil, only surpassed by cardiovascular diseases.1 Cancer is currently considered one of the most significant public health issues and has been increasing in both incidence and mortality in recent years.2 According to Bray et al.3, there were 18 million new cases and 9.6 million deaths of cancer worldwide in 2018. In Brazil, in the 2020-2022 triennium, 625,000 new cases of cancer are expected.2
Due to the aggressiveness inherent to cancer treatment, patients can present a series of complications, both systemic and local; among these, the mouth may present a number of these intricacies.4 In addition to oral mucositis (OM), the dental foci are one of the main causes of systemic infections, since the colonizing microorganisms of the oral microbiota can migrate through the surface of the epithelium and reach the circulation due to loss of epithelial integrity.5,6 Consequently, these sources of infections need to be treated or at least controlled before the patient is placed on antineoplastic therapy since the complications generated thereby can imply an increase in morbidity, mortality, and hospitalization costs.4,7,8 Thus, the objective of this article is to present the dental procedures necessary for the prevention, diagnosis, and treatment of oral toxicities of cancer treatment.
This is a narrative literature review, regarding the role of the dental surgeon in the prevention, diagnosis, and treatment of oral toxicities related to cancer treatment. A comprehensive and random search was performed for scientific articles published on the subject in question. Articles available in the Pubmed, Scielo, Cochrane, and Google Scholar databases were investigated in Portuguese and English, without a time interval specification.
The research was conducted using different combinations of the following keywords: “surgery”, “chemotherapy”, “hematopoietic stem cell transplantation”, “antiresorptive medications”, “radiotherapy”, “oral toxicities”, “dental management” and “cancer patients”. Full articles that addressed the theme, published in scientific journals, without restriction as to the methodology were included. However, book chapters and abstracts of meetings were excluded.
The search resulted in 100 selected articles, consisting of 3 systematic reviews with meta-analysis, 9 systematic reviews, 48 literature reviews, 3 randomized clinical trials, 4 prospective cohorts, 12 retrospective cohorts, 5 cross-sectional studies, 2 case-control studies, 5 case reports, 7 expert consensus panels, 1 letter to the editor and 1 qualitative study, published between 1976 and 2020.
The articles approach dental care for patients who will be, are being, or have been subjected to surgery and radiotherapy (RT) for head and neck (H&N) cancer, chemotherapy (CT), hematopoietic stem cell transplantation (HSCT), and the use of antiresorptive medications. Dental procedures were classified according to the time of treatment: prior, during, or after each therapeutic modality addressed, when applicable.
Dental care for patients undergoing head and neck surgery
Surgery is the main treatment employed for H&N tumors, usually indicated for initial tumors, but in some cases, replaced by isolated RT.9 It is reserved for tumors in which resection with neoplasia-free margins and minimal functional disorder is possible, thus ensuring complete removal of the neoplasia;10 even so, it can result in aesthetic disfigurement and significant functionality loss, which can implicate in emotional changes and social interaction impairment.9,11 Any H&N surgery patient must undergo a prior dental checkup.12–14 This assessment must include oral hygiene instruction, the removal of infectious foci, and the production of a transurgical palatal obturator, for maxillary surgeries (Table 1).
|
|
Pre-surgery management |
Late post-surgery management |
|
Oral hygiene instruction |
The patient must use an extra soft brush a, fluoride toothpaste,79 and 0.12% chlorhexidine-based mouthwashes40 in the immediate post-surgery period |
Tooth brushinga with a soft brush and fluoride toothpaste79 |
|
Oral environment adequacy |
Treatment of mucosal infections80 and elimination of any source of trauma |
Diagnosis of recurrent lesions15 |
|
Periodontal treatment |
Dental scaling according to conventional dental standards12,81,82 |
According to conventional dental standards82 |
|
Restorative treatment |
Restoration of caries lesions; adjusting or replacing pre-existing restorations12,83 according to conventional dental standards, of teeth that are not in the tumor area |
|
|
Endodontic treatment |
Endodontic treatment according to conventional dental standards,82 of teeth that are not in the tumor area |
According to conventional dental standards82 |
|
Surgery treatment (teeth or roots extraction, intraosseous lesions, periodontal surgery, implants) |
According to conventional dental standards, to remove foci of infection,84 in areas that are not affected by the tumor; installation of osseointegrated implants for future prosthetic rehabilitation13 in areas that are not affected by the tumor |
According to conventional dental standards82 |
|
Prosthetic treatment |
Transurgical palatal obturator for maxillary surgeries;14 perform pre-prosthetic planing83 |
Maxillofacial prosthetic rehabilitation via a definitive palatal obturator adapted mandibular or maxillofacial prosthesis17,18 |
Table 1 Dental care for patients undergoing head and neck surgery
aTooth brushing frequency: upon waking, before bed, and after all meals
Subsequently, these patients can be treated according to conventional dental standards; at follow-up appointments, it’s important to pay attention to recurrent or new lesions that may develop in the oral cavity15,16 (Table 1). In the context of surgeries involving the jaws, it is crucial to highlight the importance of definitive rehabilitation, to allow their reintegration into society and their aesthetic standards.17,18 In this sense, the maxillofacial prosthetics palatal obturator works as an aid in supporting the tissue, improving healing, reducing contamination and the possibility of infections; it also reduces the need for a nasoenteral tube, since it allows the patient to swallow efficiently and also an early initiation of oral feeding.19 Maxillary and mandibular adapted prostheses are essential to rehabilitating function and aesthetics. Its manufacture must follow the basic principles of dental prosthesis, which occasionally can be modified to adapt to an unusual anatomy20 and intraosseous implants may be required for better retention and stability.21 The maxillofacial prosthesis is constituted of synthetic material and molded according to specific surgical defects, allowing the repair of deformities, contributing to the aesthetic and functional rehabilitation, and the consequent return of patients' self-esteem.17 An example can be observed in Figure 1.
Dental care for patients undergoing head and neck radiotherapy
RT is a treatment modality for malignant tumors whose therapeutic agent is ionizing radiation, responsible for promoting ionization in the environment where it occurs. Most patients undergoing RT receive a total dose of 50-70Gy as a curative dose. These doses are divided into a period of 5-7 weeks, once a day, 5 days a week, with a daily dose of approximately 2Gy.22 Dental assessment before RT in patients with H&N cancer aims to minimize oral manifestations.23 The patient must undergo a physical examination, including periodontal analysis through probing and a radiographic examination.23 Dental planning must be programmed in a joint analysis with the estimated radiation dose for each oral site (Table 2).23
|
|
Pre-RT management for intraoral fields to be irradiated with doses >40Gy |
Management during RT |
Post-RT management for intraoral fields irradiated with doses >40Gy |
|
Oral hygiene instruction |
Tooth brushinga with a soft brush and fluoride toothpaste79 |
Tooth brushinga with an extra soft brush and fluoride toothpaste;79 0.12% chlorhexidine-based mouthwashes40 |
Tooth brushinga with a soft brush and fluoride toothpaste;79 use a tray with 2% neutral fluoride, 5 minutes, every day before sleep37,38 |
|
Oral environment adequacy |
Treatment of mucosal infections and elimination of trauma source16,85 |
Treatment of acute oral toxicities: dysgeusia, oral mucositis, bacterial, viral, and fungal infections, xerostomia, and pain24 |
Treatment of chronic oral toxicities: fibrosis of the oral mucosa, radiation caries, functional sequelae, xerostomia, ORN, and trismus;24 diagnosis of recurrent lesions |
|
Periodontal treatment |
Dental cleaning and scraping according to conventional dental standards are indicated to teeth with periodontal pockets measuring 4-5mm86 |
According to conventional dental standards, avoiding bone manipulation, to reduce ORN risk88 |
|
|
Restorative treatment |
Restoration of minor caries lesions, according to conventional dental standards;16 adjusting or replacing pre-existing restorations |
Preferably with glass ionomer cement due to fluoride release; radiation-induced changes in enamel and dentine may compromise bonding of adhesive materials, therefore, composite restorations often experience loss of retention and caries recurrence87 |
|
|
Endodontic treatment |
Indicatedb to teeth with periapical radiolucent lesion without symptoms (with or without previous endodontic treatment); pulp without vitality with or without symptoms and without periapical radiolucent lesion86 |
Teeth with conventional indications and root debris for burial with antibiotic prophylaxis during the endodontic treatment; the instrumentation, irrigation, drying, and medication of the canal must be monitored accurately; avoiding caustic substances to avoid inflammation; do not leave the tooth open "to drain"89 |
|
|
Surgery treatment (teeth or roots extraction, intraosseous lesions, periodontal surgery, implants) |
Extractionc of dental elements with deep caries in which excavation can lead to pulp exposure, extensive periapical lesion seen on radiographs combined with periodontal disease in symptomatic teeth, teeth with periodontal pockets ≥ 6mm, teeth with gingival recession ≥ 6mm, teeth or roots partially impacted or with radiographic abnormalities such as periapical lesions and teeth with internal or external resorption;23 removal of intraosseous lesions.86 Periodontal surgery should only be performed if there is enough time to complete the reabilotation procedure; implnats should only be performed if there is enough time to complete osseointegration" |
Not indicated; if necessary, they should be performed with minimal trauma by a specialist87 |
Should be avoided due to the risk of ORN; given the absolute indication for surgical procedures with bone manipulation, the minimally invasive surgical technique should be employed, that is, avoiding osteotomy and seeking primary synthesis, as well as assessing the need for antibiotic prophylaxis; mouthwash with chlorhexidine should be used preoperatively and postoperatively until the healing of the extraction wound is observed79,90 |
|
Prosthetic treatment |
Removable prostheses with jagged or rough edges that may implicate trauma should be adjusted90 |
Not indicated, and the patient should only use removable prostheses, for food or social situations88 |
Generally, removable prostheses should be avoided, due to trauma; they can be used only for aesthetics or function87 |
Table 2 Dental care for patients undergoing head and neck radiotherapy
RT, radiotherapy; ORN, osteoradionecrosis
aTooth brushing frequency: upon waking, before bed, and after all meals;
bShould only be performed if there is enough time to complete the procedure and for the apex lesion to regress until the beginning of the RT, otherwise extraction should be recommended;86
cThere must be 21 days between the extraction and the beginning of the RT, to guarantee the initial healing of the oral tissues.23
During RT, routine dental treatment is not indicated; only evaluation and resolution of dental emergencies should be performed (Table 2). The dental surgeon must diagnose and treat the RT acute oral toxicities24 (OM25–27, dysgeusia,12,22,28,29 infections, pain, and xerostomia) (Table 3), that implicate an inability to eat, speak, clean, increased costs with hospitalization, and reduced quality of life.30–33
|
|
CT |
HSCT |
RT |
Management |
Observations |
|
Acute oral toxicities |
|
|
|
|
|
|
aGVDH |
No |
Yes |
No |
Mouthwashes with corticosteroids (dexamethasone (0.1mg/mL) and clobetasol (0.5mg/mL) can be used91 |
It is not a usual manifestation |
|
Bacterial, viral, and fungal infections |
Yes |
Yes |
Yes |
Prescription of specific medications according to the responsible pathogen |
Infections usually present atypical clinical features in immunosuppressed patients (CT and HSCT); in the cases of fungal infections, treatment with nystatin solution12,28 or fluconazole79,92 are recommended |
|
Dysgeusia |
Yes |
No |
Yes |
Nutritional care, constant water intake, and use of salivary substitutes are recommended12,93 |
In a recent study, the use of zinc-L-carnosine demonstrated encouraging results in the treatment of dysgeusia in these patients94 |
|
Oral mucositis |
Yes |
Yes |
Yes |
Application of low-power laser (660nm, 8J/cm2) from the appearance of oral mucositis until healing95 |
Add dexamethasone mouthwash (mildest OM by mTOR inhibitors;54,55 and OM by tyrosine kinase inhibitors EGFR/ HER1, Pan-HER or EGFR-inhibiting monoclonal antibody55); clobetasol gel or cream, or intralesional triamcinolone injection (severe OM by mTOR inhibitors);55 dose adjustment or treatment interruption, and high-dose systemic corticosteroids, should be considered in line with the oncologist (unresponsive cases)55 |
|
Pain |
Yes |
Yes |
Yes |
Opioids prescription;53 associated with OM, should be managed with topical 0.2% morphine prescription96 |
|
|
Neurotoxicity |
Yes |
No |
No |
Prescription of opioids or, if they don’t work, anticonvulsants, antidepressants, corticosteroids, and anesthetics, as well as nutritional supplementation with alpha-lipoic acid, vitamin E, erythropoietin, and acetyl-L-carnitine53 |
|
|
Xerostomia |
Yes |
Yes |
Yes |
Use of salivary stimulants (mechanical or gustatory), salivary substitutes, or systemic agents12,24 |
|
|
Chronic oral toxicities |
|
|
|
||
|
cGVDH |
No |
Yes |
No |
Prescription of high-potency topical corticosteroids, calcineurin inhibitors, and analgesics;97 use of artificial saliva, oral rinses, sugar-free candies, sialogougues71 |
|
|
ORN |
No |
No |
Yes |
It is still considered a challenge and we still do not have scientific evidence regarding the best treatment98–100 |
Prescription of antibiotics, anti-inflammatories, pentoxifylline associated with tocopherol, hyperbaric oxygen chamber sessions, ultrasound, and surgical procedures for removing the necrotic bone tissue associated or not with the graft could be considered101–103 |
|
Pain |
No |
Yes |
Yes |
Opioids prescription53 |
|
|
Radiation dental caries |
No |
No |
Yes |
The removal of carious tissue is recommended through manual curettes and the use of cariostatic substances, with the subsequent restoration of the elements with resin glass ionomer35,104 |
|
|
Second primary tumor |
Yes |
Yes |
Yes |
Perform biopsy to diagnose and refer the patient to the oncologist for new cancer treatment71 |
|
|
Trismus |
No |
Yes |
Yes |
A strict regimen of oral exercises associated with physiotherapy is recommended to minimize this complication85 |
|
|
Xerostomia |
Yes |
Yes |
Yes |
Use of salivary stimulants (mechanical or gustatory), salivary substitutes, or systemic agents12,24 |
|
Table 3 Management of acute and chronic oral toxicities from antineoplastic therapy
CT, chemotherapy; HSCT, hematopoietic stem cell transplantation; RT, radiotherapy; aGVDH, acute graft versus host disease; cGVDH, chronic graft versus host disease; ORN, osteoradionecrosis
After completion of RT, the patient must be closely monitored to identify and manage possible complications. At first, dental follow-up appointments should be monthly, changing to quarterly after most acute toxicities are controlled. The dentist's actions must be preventive, to promote oral health, and procedures that involve bone manipulation (such as extractions and dental implants) should be avoided due to the risk of osteoradionecrosis (ORN)24 (Table 2). Moreover, the chronic oral toxicities, which are a limited opening of the mouth (trismus) associated with mucosal fibrosis,27 pain in the mucous membranes, second primary tumors, xerostomia,12,32,34 radiation-induced dental caries35–38 and ORN,30,32,39–41 should be diagnosed and treated (Table 3). Cases of radiation-induced dental caries and ORN are shown in Figures 2 and 3, respectively. Considering that RT is a local treatment, a dentist should, as previously stated in the surgery section, pay attention to possible recurrence, identifying, and forwarding to the oncologist as soon as possible for better management.
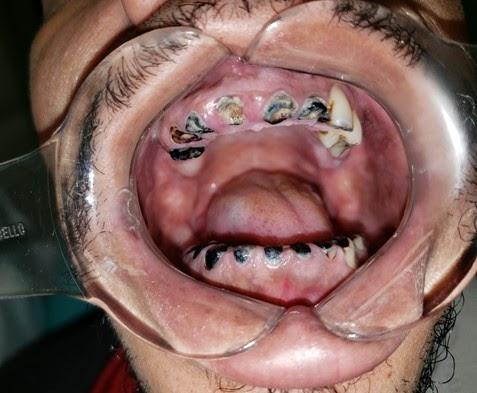
Figure 2 Intraoral aspect of a patient with generalized dental involvement due to radiation-induced dental caries after RT treatment.
Dental care for patients undergoing chemotherapy
CT is the systemic treatment that uses antineoplastic drugs in regular intervals, according to the therapeutic regimen.42 It can be used in association with other treatment modalities, in adjuvant (to avoid tumor spread) or in neoadjuvant (to stimulate the reduction of the tumor), thus optimizing surgery or RT.43 The therapeutic scheme of choice relies on histopathological and molecular diagnosis, clinical condition, functional capacity, and patient preference; monotherapy or polychemotherapy can be used.44,45
Dental planning before CT may be more conservative when compared to the one for RT or HSCT. The prior dental evaluation must consist of an oral hygiene instruction, elimination of caries, periodontal disease, endodontic infections, and other possible foci of infection in the oral cavity that may be exacerbated during the neutropenic stage expected for these patients (Table 4).46
|
|
Pre-CT management |
Management during CT |
Post-CT management |
|
Oral hygiene instruction |
Tooth brushinga with a soft brush and fluoride toothpaste79 |
Tooth brushinga with an extra soft brush and fluoride toothpaste;79 0.12% chlorhexidine-based mouthwashes40 |
Tooth brushinga with a soft brush and fluoride toothpaste51,79 |
|
Oral environment adequacy |
Treatment of mucosal infections51,105 and elimination of any trauma source,46 such as orthodontic device |
Treatment of acute oral toxicities: oral mucositis, dysgeusia, xerostomia, bacterial, viral, and fungal infections40,47–50 and neurotoxicity27,51 |
Diagnosis of recurrent and metastatic lesions;56–59 check for infections (viral and fungal)51 |
|
Periodontal treatment |
Dental cleaning and scraping according to conventional dental standards46,51 |
Necessary dental care, always considering laboratory exams90,106 |
Need for routine systematic oral hygiene107 |
|
Restorative treatment |
Restoration of minor caries lesions; adjusting or replacing pre-existing restorations40,46,51,105 according to conventional dental standards |
Necessary dental care, always considering laboratory exams90,106 |
According to conventional dental standards107 |
|
Endodontic treatment |
Endodontic treatment of severe caries, pulp involvement, dental abscess, and apical periodontitis (symptomatic lesions and lesions ≥5 mm)40,46,51,105, if the patient's systemic conditions allowb |
Necessary dental care, always considering laboratory exams90,106 |
According to conventional dental standards107 |
|
Surgery treatment (teeth or roots extraction, intraosseous lesions, periodontal surgery, implants) |
Extraction of teeth with apical periodontitis, pocket depth (≥6mm), furcation I, II or III, partially impacted teeth, root debris, intraosseous lesions, radiographic abnormalities such as root resorption and mobile primary teeth with >50% root resorption46 if the patient's systemic conditions allowc |
Necessary dental care, always considering laboratory exams90,106 |
According to conventional dental standards,107 as long as the blood count and biochemical tests allow, exceptd for patients using bevacizumab108 |
|
Prosthetic treatment |
Denture fitting should be checked, with readjustment or removal of those prostheses that cause trauma51,107 |
Not indicated, and the patient should only use removable prostheses, for food or social situations79 |
According to conventional dental standards,105 but the best time to perform the procedure should be discussed and agreed upon with the responsible oncologist51 |
Table 4 Dental care for patients undergoing CT
aToothbrushing frequency: upon waking, before bed, and after all meals;
bShould only be performed if there is enough time to complete the procedure and for the apex lesion to regress until the beginning of the chemotherapy, otherwise extraction should be recommended;51
cAt least 2 weeks before chemotherapy starts, so that there is time for healing;51
dPatients using bevacizumab have difficulty in healing, therefore, in these cases, a 28 days interval after drug interruption should be given before performing surgery and further 28 days to restart the drug.108
Given CT is a systemic therapy, during treatment, especially in the period of immunosuppression, it may cause changes in the oral mucosa that may lead to a direct impact on the patient's quality of life, occasionally leading to treatment interruption.47 Therefore, during CT, dental management should consist of emergency procedures - always considering laboratory exams (Table 4)- and the management of acute oral toxicities (OM, dysgeusia, xerostomia, bacterial, viral, and fungal infections40,47–50 and neurotoxicity27,51) (Table 3).
It is important to note that some CT drugs are more toxic to the tissues, being frequently associated with OM, such as methotrexate, 5-fluorouracil, cyclophosphamides, cisplatin, and purine analogs such as cytarabine.52,53 Regarding OM caused by mTOR inhibitors,54,55 tyrosine kinase inhibitors EGFR/HER1, Pan-HER, or EGFR-inhibiting monoclonal antibodies,55 they have distinct clinical presentations and specific management (Table 3).
Considering that acute effects related to CT tend to cease soon after the organism's recovery, the patient undergoing CT does not need long-term post-treatment care that would differ from those dispensed in routine annual dental consultations (Table 4). Despite that, the dentist should pay attention to possible metastatic lesions that may manifest in the oral cavity.56 These manifestations are rare in this area, indicate advanced disease progression,57,58 and imply a poor prognosis when it comes to survival.57,59 The identification and early management of these can significantly impact the management (thought palliative treatment-seeking maintenance of oral function and management of pain) and patient’s quality of life.57,58
Dental care for patients undergoing hematopoietic stem cell transplantation
HSCT is the therapeutic modality for hematological diseases' treatment, whether benign or malignant, hereditary or acquired - among them are leukemias, lymphomas, and multiple myeloma.60 It consists of providing the patient with hematopoietic stem cells that will be lodged in the bone marrow, to re-establish their function.61 HSCT can be autogenic, allogeneic, or syngeneic and the progenitor cells may come from bone marrow, peripheral blood, or umbilical cord.62
Dental care before HSCT should be performed on all patients to minimize the complications during treatment,46 according to Table 5. During the HSCT, acute oral toxicities62 (infections, OM,63,64 xerostomia63 and acute graft versus host disease (aGVHD))65,66 must be controlled (Table 3) and emergency procedures performed,51 always considering laboratory exams (Table 5). Figure 4 illustrates one of the possible clinical presentations of aGVHD.
|
|
Pre-HSCT management |
Management during HSCT |
Post-HSCT management |
|
Oral hygiene instruction |
Tooth brushinga with a soft brush and fluoride toothpaste79 |
Tooth brushinga with an extra soft brush and fluoride toothpaste;79 0.12% chlorhexidine-based mouthwashes40 |
Tooth brushinga with a soft brush and fluoride toothpaste79 |
|
Oral environment adequacy |
Treatment of mucosal infections and elimination of any trauma source46 |
Treatment of acute oral toxicities: infections, oral mucositis, xerostomia, and acute graft versus host disease62 |
Treatment of chronic oral toxicities: xerostomia,51,63 chronic graft versus host disease;51,65 Diagnosis of second primary tumors,70 recurrence and metastasis lesions |
|
Periodontal treatment |
Dental scaling according to conventional dental standards46,51 |
Only emergency procedures, always taking laboratory tests into account51 |
Dental disease should be considered a source of infection and therefore should be managed routinely, avoiding invasive treatment. Removal of dental plaque deposits using an ultrasonic scaler should be minimized if the patient is immunosuppressed51 |
|
Restorative treatment |
Restoration of minor caries lesions; adjusting or replacing pre-existing restorations40,46,51,105 according to conventional dental standards |
Only emergency procedures, always taking laboratory tests into account51 |
Dental disease should be considered a source of infection and therefore should be managed routinely, avoiding invasive treatment. Procedures that generate aerosols that may lead to aspiration, should be minimized if the patient is immunosuppressed51 |
|
Endodontic treatment |
Endodontic treatment of severe caries with pulp involvement, dental abscess, and apical periodontitis (symptomatic lesions and lesions<6 mm)40,46,51,105, if the patient's systemic conditions allowb |
Only emergency procedures, always taking laboratory tests into account51 |
Dental disease should be considered as a source of infection and therefore should be managed routinely51 |
|
Surgery treatment (teeth or roots extraction, intraosseous lesions, periodontal surgery, implants) |
Extraction of teeth with apical periodontitis, pocket depth (≥6mm), furcation I, II or III, partially impacted teeth, root debris, intraosseous lesions, radiographic abnormalities such as root resorption and mobile primary teeth with >50% root resorption46 if the patient's systemic conditions allowc |
Only emergency procedures, always taking laboratory tests into account51 |
Dental disease should be considered as a source of infection and therefore should be managed routinely51 |
|
Prosthetic treatment |
Denture fitting should be checked, with readjustment or removal of those prostheses that cause trauma51,107 |
Not indicated, and the patient should only use removable prostheses, for food or social situations51 |
According to conventional dental standards105, but the best time to perform the procedure should be discussed and agreed upon with the responsible oncologist51 |
Table 5 Dental care for patients undergoing HSCT
aToothbrushing frequency: upon waking, before bed, and after all meals;
bShould only be performed if there is enough time to complete the procedure and for the apex lesion to regress until the beginning of chemotherapy, otherwise extraction should be recommended;51
cAt least 2 weeks before chemotherapy starts, so that there is time for healing.51
The patient should be maintained in control via a semiannual consultation program or following specific needs after the immediate HSCT and annual control in late HSCT stages. In these consultations, the dentist must plan dental treatments according to Table 5 and diagnose and/or treat chronic toxicities such as xerostomia,63 trismus, chronic graft versus host disease (cGVHD),65,67 and second primary tumor66,68–70 (Table 3). A case of cGVHD is represented in Figure 5. As previously reported in the CT section, the dentist should also pay attention, in the follow-up appointments, to oral lesions that could indicate metastasis from other tumors manifesting in the oral cavity. Patients with Fanconi anemia undergoing HSCT should be scheduled for control visits every 6 months, due to the increased risk of developing squamous cell carcinomas in the oral cavity.71
Dental care for patients in the use of antiresorptive medications
In the oncological context, it is necessary to consider patients that use bisphosphonates (BPs) intravenous (IV) or orally or denosumab due to the association of these drugs with medication-related osteonecrosis of the jaws (MRONJ). Despite the low incidence, MRONJ causes an abrupt drop in patient’s quality of life.72 In this same context, it is important to also consider the use of antiangiogenic drugs, as this association increases the risks of developing MRONJ.73
BPs are synthetic pyrophosphate-like drugs, which bind tightly to hydroxyapatite and reduce metabolism and bone remodeling. They are medications with a long half-life and thus remain in the bone tissue for more than 10 years after treatment interruption.73 The main BPs associated with MRONJ cases are zoledronate, pamidronate, IV ibandronate, alendronate, oral ibandronate, risedronate, and clodronate.74 These drugs are prescribed for patients at risk/evidence of bone metastasis, or for the treatment of multiple myeloma, Paget's disease, and disorders of calcium metabolism.75–77
Denosumab is a monoclonal antibody that binds to the receptor-ligand responsible for the activation of the nuclear factor kappa B ligand (RANK-L), thus blocking the maturation, function, and survival of osteoclasts. It is also prescribed for patients presenting osteoporosis or risk/evidence of bone metastasis. Unlike BPs, this medicine has a half-life of 25 to 32 days.73
The dentist is responsible for investigating the use of these drugs during anamnesis, both in cancer patients and in the general population, before defining the dental treatment plan. The ideal is that before starting the use of antiresorptive medications, patients should be evaluated and prepared by the dentist to minimize the subsequent need for invasive treatment,72 and the patient should be guided and managed similarly to those subject to RT for H&N tumors77 (Table 2).
Patients using antiresorptive medications should be scheduled for a control visit every 6 months. Dental management is also similar to that employed after RT (Table 2), to maintain the status of adequate oral hygiene and perform an early diagnosis of MRONJ and eventual metastatic manifestations in the oral cavity. The MRONJ management is still controversial and can include non-surgical procedures, such as pain and infection control (oral hygiene care, periodontal disease management, chlorhexidine gluconate 0.12% mouthwash, antibiotic therapy, and systemic corticosteroid therapy) and surgical treatment when necessary.72
After the start of therapy, given the absolute indication for surgical procedures with bone manipulation, the minimally invasive surgical technique should be employed, that is, avoiding osteotomy and seeking primary synthesis, as well as assessing the need for antibiotic prophylaxis; mouthwash with 0,12% chlorhexidine should be used preoperatively and postoperatively until healing; suspension of therapy according to each case should be assessed.73,75,77,78 For patients who have had oral BP for less than 4 years and do not have associated risk factors, changes in surgical planning are not necessary. On the other hand, for patients using oral BP associated with corticosteroids and antiangiogenics, also for less than 4 years, it is suggested that the BP should be interrupted for 2 months before extractions until full bone healing. Likewise, it should be discontinued in patients treated for more than 4 years, that is, 2 months before the procedure until full bone healing. The maneuvers for suspending and resuming drug use must be agreed with the oncologist responsible for conducting the patient's treatment.77
Regarding the interruption of IV BPs, data in the literature is still scarce, and there is no established guideline. However, in cases of MRONJ, the oncologist may consider interrupting antiresorptive therapy until soft tissue is fully healed over the jawbones, depending on the status of the disease. Regarding denosumab, it was observed that its antiresorptive effects dissipate approximately within 6 months after discontinuing the drug, however, there are no studies to support or refute this strategy for prevention or treatment of MRONJ, making further studies necessary.77
It is also important to consider the use of antiangiogenics, prescribed mainly for cancer patients for inhibiting several mechanisms involved in tumor neoangiogenesis. In this group of drugs, bevacizumab (inhibitor of vascular endothelial growth factor), sunitinib (tyrosine kinase inhibitor), and everolimus (inhibitors of the target protein of rapamycin in mammals) stand out. These medicines do not tend to accumulate in the bone and their half-life range from 30 hours for everolimus to 20 days for bevacizumab, varying according to the medication.73 It is important to note that the benefits versus risks of discontinuing therapies must be calculated jointly with the oncologist and the patient,78 according to their systemic conditions.
The consulted articles were unanimous regarding the need for dental care in pre, trans, and post-treatment cancer patients, showing the importance of including specialized dental surgeons in the oncology care team. In this way, the dentist must act in the direction of prevention, early diagnosis, and treatment of oral toxicities. The dentist is responsible for minimizing its consequences and improving the quality of life of patients.
None.
The authors declare that there is no conflict of interest.

©2021 Alves, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.