Journal of
eISSN: 2373-4426


Mini Review Volume 14 Issue 1
FRCS, FRCP, PGCMedEdu, United Kingdom
Correspondence: Ian Munro Rogers, FRCS, FRCP, PGCMedEdu, 21 Strathview Place, Comrie, Perthshire PH62HG, Scotland, United Kingdom
Received: March 16, 2024 | Published: April 10, 2024
Citation: Rogers IM. Neonatal acidity and pyloric stenosis of infancy (PS) - the beginning and the end two stories. J Pediatr Neonatal Care. 2024;14(1):75-78. DOI: 10.15406/jpnc.2024.14.00545
The author tells two stories.
Story one describes the beginnings of his research interest into the cause and effect of neonatal acid secretion. It also explains the new proposal that an insensitivity of the usual negative feed-back in adults between neonatal gastrin and gastric acidity is not functioning at birth and takes some weeks to develop. Because of this, gastrin levels and acidity rise together from birth. This means that when feedback maturity occurs, there will be a temporary peak in both gastrin and gastric acidity before mutual restraint is established.
The early gap in acid defense is filled by functional maternal gastrin transfer during labor which produces an external source of acid secretion until the neonatal gastric mucosa has matured.
Story two relates the effects of these earlier changes to the baby who has inherited an enhanced parietal cell mass. Such babies develop a critical hyperacidity during developmental peak acidity. Acidity- provoked sphincter contractions; sphincter hypertrophy and gastric outlet obstruction (GOO) may then supervene.
All the bewildering clinical features are understandable within the framework of this hypothesis.
The beginning of this first story is quite definite. The year is 1972- the location is the Pediatric Surgical wards in Stobhill Hospital, Glasgow, Scotland and the dramatis persona are me, a lowly rotating surgical registrar- the late and much missed John Grant Consultant Pediatric Surgeon and Ian Drainer a Senior Registrar in Pediatric Surgery.
A point of developing interest in Pediatrics at that time, was the trans-placental exchange of active biochemical compounds especially during labor. The process of Rhesus immunization of mother against her child was a good example but there were others.
Similarly, duodenal ulcers in Glasgow and elsewhere were very common. Gastrin, as a potential cause of the associated hyperacidity in these patients, was much investigated.
That year I discovered an old paper published in 1941 by an anesthetist from Texas, U.S.A.—then working in the Royal Infirmary, Edinburgh called Robert Arden Miller.1
Neonatal hyperacidity
The phenomenon and the cause
Miller had discovered a wave of temporary neonatal hyperacidity soon after birth and thought that an acid producing chemical was being transmitted from mother to baby during delivery (Figure 1). It would take 25 years or more before these findings were confirmed.2,3 The extraordinary thing is that in 1941 gastrin and its effect was yet to be discovered.
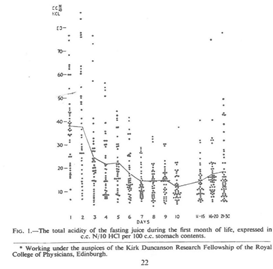
Figure 1 This graph shows an early wave of gastric acidity after the first gastric aspiration followed by beginnings of more acidity from Day 10.
Miller had also noted a later gradual rise in acidity at around 10 days which he thought was caused by the neonatal gastric mucosa progressively maturing.1
Now in 1974 we had a chance to settle the matter. Was gastrin transfer really involved?
We tried but failed to confirm supportive evidence of maternal transfer.
However, we found that the 4th day fasting gastrin level was steeply elevated (several times higher than the normal fasting adult level) compared to the cord level at birth.
Cord baby gastrins were higher in the spontaneous labors compared to labors induced by syntocinon. Furthermore, there was a highly significant individual correlation between the day 1 level and the level at day 4 only in the syntocinon labors (Figure 2).4
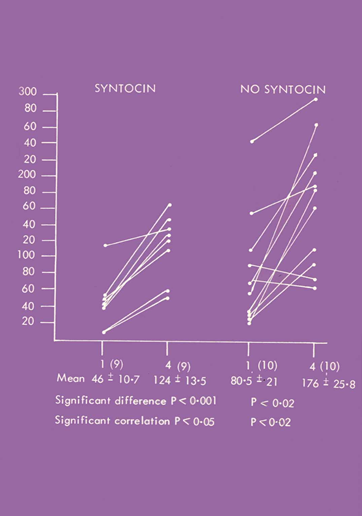
Figure 2 19 births were investigated, of which 9 were induced by syntocinon. Day 4 fasting gastrin levels were elevated. The Day 4 and Cord levels were only individually correlated in the syntocinon babies.
Because of the short half-life of gastrin, we presumed by day 4 gastrin would be entirely neonatal in origin. Thus, the highly significant individual correlation between Day 4 and cord blood suggested that syntocinon cord gastrin 1 level must largely consist of neonatal gastrin as well.4
We imaginatively proposed that the higher cord gastrins in spontaneous labors might reflect a maternal contribution only in spontaneously born babies. In 1995 Singlit and others showed that spontaneously vaginally delivered piglets had higher gastrins and higher gastric acidity at birth than those delivered by caeserian section.5 The possibility of maternal gastrin transfer only during labor as the cause was not considered.
We tried to show this effect in human babies but only managed to confirm that the gastric pH 4 hours after birth was more acid than at birth.3
The supportive evidence however for maternal functional gastrin transfer is easy to find. Human maternal gastrin similarly rises progressively with gestation reaching a peak at labor and falling sharply within hours of birth.6
Functional maternal gastrin transfer in dogs has now also been clearly demonstrated.7 Miller’s insight in this process had been correct.
Neonatal hypergastrinemia in the fed baby has now been confirmed many times. When the feed-back matures gastrin (and acidity) falls as they come under mutual restraint. There is no post-feed rise in gastrin before maturity when gastrins are already being maximally stimulated. Only when they become lower is a post-feed gastrin rise detectable.8,9,10
These same changes occur in dogs which in addition show no acid response to pentagastrin before maturity. Only after maturity can an acid response to pentagastrin be detected.9
The investigators who reported these findings did so as observational studies only-not in the framework of an insensitive feed-back at birth hypothesis-thus adding to the significance of their findings.
When basal acid secretion and pentagastrin-stimulated peak acid secretion are compared in normal newborns, there is no difference between the acid secretion rates between Day 1 and Day 2. It is not possible for pentagastrin to increase acid secretion when native gastrin is already stimulating the parietal cell maximally.11
The anticipated maturation of the negative feed-back is validated by the graph produced by Agunod which shows a peak in acid, intrinsic factor and pepsin- at 17 days. After this, the beginnings of negative feed-back for the first time, introduces mutual restraint and these values fall.12
Gastrin, whose secretion is stimulated by feeds, has a trophic (nutritional) effect on the gastric mucosa. Hence in the fed baby, acid, intrinsic factor and pepsin all increase with natural gastrin stimulation.12 There is no gastrin rise when the baby is not fed.10
Miller’s original observation of early temporary hyperacidity fills the gap in acid defense while awaiting mucosal maturity. Such a temporary system avoids long-term acid problems in normal human development.
It is dynamic and neat.
The pyloric stenosis story (PS)
The Primary hyperacidity hypothesis, the beginning of this second story is less precise. It took its own time.
It was clear that gastric acidity, with its effect of contracting the sphincter, was likely to be involved.13,14
Would this be caused by an abnormality in gastrin and would the known hyperacidity be a secondary effect?
The consequence of continual milk feeding of the hyperacid and consequently hungry baby, would produce a distended buffered neutral antrum- classical conditions in which even more antral gastrin would be secreted-and so on---. It is of interest in this context that PS babies share the same preponderance of blood group A-the blood group associated with inherited hyperacidity with adult duodenal ulcer patients.15,16
The hypothesis
When we measured fasting plasma gastrin in IHPS and matched controls, there was no significant difference. However as expected, the fasting gastric aspirate had a greater volume and was more milk stained than the matched controls. Despite that the aspirate more acidic in PS.17
We were now beginning to realize that a much simpler explanation made more sense.
What if PS babies developed the condition because of an above average inheritance of parietal cells? Viewed from this stand point everything became clear. All the otherwise strange clinical features which bewildered us and bewildered your readers were now all easily explained.
The pioneering paper by the late Prof. John Dodge made our change in focus even more pointed. He showed that PS was produced in new-born puppy dogs by giving penta-gastrin injections to their mothers before delivery. A greater frequency of PS resulted if the puppies also received the injections directly. These puppies would undoubtedly have been suffering from (penta) gastrin-induced gastric hyperacidity.18
Our subsequent experiments confirmed that PS babies did indeed have gastric hyperacidity (Figure 3).19 Others have shown that this hyperacidity persists 1 week after successful pyloromyotomy20 and that the consequences of hyperacidity are more frequent in adult life post-pyloromyotomy. A retained normal acid secretion was NOT the cause.
The subsequent discovery that male pre-term babies produced more acid than females, made us even more convinced we were on the right lines.21
The classical “big, bouncing baby boy” description of the baby most likely to develop PS is no mean analogy.
Big mature babies as Miller also hypothesized1 have better developed gastric mucosa; they are consequently likely to have more acid; the greater the acidity-the greater their hunger; the greater the hunger- the more frequently they feed; the more they feed-the more frequent and vigorous are the sphincter contractions. Sphincter hypertrophy and gastric outflow obstruction average are an ever-present danger.
PS babies do have birth weights greater than the average weight;22 more frequent feeding makes PS babies present earlier23 and the act of feeding itself induces frequent and the most vigorous high amplitude sphincter contractions.24
Similarly, novice mothers with first-born babies in their enhanced anxiety are more likely to continue to feed their vomiting baby; the summer peak incidence of PS will be caused by heat dehydrated babies feeding frequently from thirst alone. Late hyperacidity problems have also been documented.25
There are no accepted clinical features in this condition which are not explained by this theory.
Diagnostic and therapeutic considerations
Medical treatment at present with atropine and controlled underfeeding with gastric washouts- does reduce acidity and does produce enduring cures The informed reader will not be surprised to know that such an enduring cure is effected by such temporary treatment. These infants continue to lose lots of acid when they vomit- and ranitidine treatment quickly restores acid-base stability pre-operatively.26
As you would expect any baby within the correct age group, who is vomiting- and has alkalosis- will invariably have PS.27 They are losing a more concentrated acid.
None.
None.
The author declares that there are no conflicts of interest.

©2024 Rogers. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.