MOJ
eISSN: 2381-179X


Case Report Volume 11 Issue 2
Department of Cardiology, Medical College Hospital, India
Correspondence: Prabha Nini Gupta, Department of Cardiology, Medical College Hospital, India
Received: April 16, 2021 | Published: April 27, 2021
Citation: Gupta PN, Velappan P, Kulkarni V, et al. A novice’s approach to a ventricular septal rupture closure with an atrial septal device. MOJ Clin Med Case Rep. 2021;11(2):53-58. DOI: 10.15406/mojcr.2021.11.00381
We describe here an approach to a ventricular septal rupture in a patient with a recent myocardial infarction. We briefly describe how we decided the route, the type of device and the procedure we followed. We also detail how we followed up the patient. We performed the VSR closure 5 days after the index infarction as the patient was in hypotension. The ventricular septal rupture was crossed with a Terumo wire covered by a right coronary guide catheter passing from the left ventricle to the right ventricle via a right femoral artery approach and the right internal jugular vein was used to form an arteriovenous loop. Using a patent ductus arteriosus sheath the ventricular septal rupture was crossed and a 16 mm Amplatzer ASD closure device was deployed. The patient had a mild residual leak and tolerated the procedure well.
We have also included a few words from various authors who have attempted VSR closure including some from 2019.
Keywords acute myocardial infarction, endovascular device closure, post myocardial ventricular septal defect
Ventricular septal rupture is a dreaded complication of acute myocardial infarction that is on the decline in the interventional era.1 This condition has very high mortality (100%) when closed by devices in the acute stage and a slightly reduced mortality in the chronic stage. The ACC still recommends early surgical closure of defects.2 These defects have a reported mortality of 80% when performed before 2 weeks, and a 30-20% mortality when done after 2 weeks of the index MI. The greater the delay, the better are the outcomes as the margins of the VSR fibrose and strengthen, so that the sutures and devices do not tear the friable tissues. Unfortunately, many relatively stable patients still suddenly die.
Other reports of ventricular septal rupture closure by devices are the following.3-6 A new algorithm claims that VSRs under 15 mm can be closed by devices. The largest VSR device is 22mm. Therefore, the largest VSR that can be closed is 15 mm. All other ruptures should be sent for surgical repair.4VSRs can be successfully closed by ASD devices as late as 100 days after myocardial infarction.5 The main considerations for ventricular septal rupture (VSR) are as follows:
Device sizing
Our VSR was 0.6cm in size. We wanted an oversized device as recommended by others.4 Risseeuw described that sizing the device depends on whether the device closure is done within 14 days of the index MI (acute phase) or during the chronic phase. He describes that early phase sizing should be at least 10 mm more than the size of the VSR to prevent tearing of the septum. In the more chronic phase, he recommends the device be only 7mm more than the defect. Authors recommend going 1.8 to 2.2 times the size of the defect as the defect can tear both during or after device implantation. Malthias recommended that the largest VSR that can be closed by a device is 1.5cm, and larger VSRs should be closed by surgery.4 We decided after consulting experts in congenital ventricular septal defects to use a 16-mm device.
Route of deployment
We decided on the jugular route with a right femoral artery approac.5 Avoiding the sub-valvular structures is easier with the jugular route. Furthermore, this route is a more direct route to mid-septal ventricular septal ruptures.
Approach to crossing the ventricular septal rupture: RV or LV route 6,7
On echocardiography the defect was tortuous on the right ventricular side. Therefore, we decided to use a left-sided approach. There are two approaches to reach the left ventricle to enable crossing to the right ventricle through the VSR. One is the transeptal approach . (atrial septal defect or through a patent foramen ovale, or by trans-septal puncture)
The second approach to the left ventricle is the aortic route. This route is through the right femoral artery through the aorta and then into the left ventricle from here the guide wire is tracked across the VSR into the right ventricle. We used this route .
Any device closure requires an arteriovenous loop to aid device closure.
We chose the right internal jugular approach for this procedure, as the VSR was in the mid-septal ventricular septum, and it is supposed that less interposition of chordae and valvular apparatus occurs with this route.
Echocardiography
Viewing the ventricular septal rupture via a transoesophageal echo (TEE) is difficult. We routinely do ASD device closure with TEE guidance, but for VSR closure, the transthoracic route (TTE ) route was used more frequently in literature.5,7
Echocardiographic views7
Dr Vijaylekshmi has described the approach to imaging the VSR. Akin to congenital VSDs if the defect is in the 12 o’clock to 11 o’clock position, it is an anterior defect.7 If it is between 11 and 9 o’clock, it is a middle defect, and if it is between 9 and 7 o’clock, it is a posterior defect.
Which device to employ?
The ideal would be, of course, a dedicated ventricular septal rupture device. This device was not available to us as it had to come from a distant town. Dr Rohit described which device to use for which type of VSD extensively.8 Basically, for defects up to 1.5cm, the Amplatzer septal occluder dedicated to ventricular septal rupture is available. For larger sizes, an atrial septal defect device is recommended. Asymmetrical devices for peri-membranous VSD closure have not yet been approved by the US FDA.Since we did not have a dedicated occluder we utilized an atrial septal defect closure device.
Atrial septal defect occluders
Atrial septal defect occluders have been tried in some cases.5 The Life Tech device is believed to be superior for VSD closure as it reduces the incidence of heart block as it is a softer device of nitinol mesh and has been shown to be useful even in peri-membranous VSD closure (congenital).9
What is the time frame in which the device should be deployed? How long can we wait?
The ACC recommends very early closure. However, many cases in the literature demonstrate successful closure 2 weeks to 3 months after the rupture has occurred, which is possibly a natural selection of better cases.
What about revascularization?
Some cases in the literature describe that attempting PCI for the infarct-related artery during the VSR closure leads to higher mortality, probably due to excess dye. However, anecdotal reports of performing PCI first and device closure later exist.5
Angiography during the procedure
An LAO 60 degree angiogram with 20 degree cranial is good for sizing the ventricular septal rupture in diastole. Authors recommend working in the LAO at 40 degrees after the initial angiogram as this setting is comfortable.
Mr G was a 55-year-old male patient, with diabetes mellitus and systemic hypertension. He presented on 4-1-2017 to our hospital.
He developed sudden onset chest pain on 2-1-17 while resting at home with radiation of chest pain to the arms and sweating. He reported to us after an interval of 48 hours of onset of the chest pain. His step sister died of CAD at the age of 48 years. He was a smoker and regularly drank alcohol.
His functional parameters were an O/E PR of 104/min and blood pressure of 80/60. His jugular venous pressure (JVP) was elevated to 6cm. He had mild cardiomegaly. His first and second heart sounds were normal, and he had a left ventricular third heart sound and a pansystolic murmur at the lower left sternal border. He also had an audible pericardial rub. He had bilateral course crepitations in the lower lung fields.
A provisional diagnosis of CAD extensive anterior wall myocardial infarction, Killip Class 4 with ventricular septal rupture and a late presentation was made.
He was started on i/v heparin, enteric-coated aspirin, clopidogrel, and dopamine. His BP was still only 70 systolic.
His electrocardiogram showed a sinus rhythm with a mean frontal QRS axis of + 90 degrees, a PR interval of 120msec, and a Q RBBB in VI with T inversion in leads V1 to V4 on 4/1/17.
His echocardiogram at presentation showed a mid-muscular VSR with a gradient of 44mmHg and a size of 0.6 cm. He also had mild TR and mild MR with an EF of 32%. His tricuspid regurgitation jet was 28mmHg.
In the short-axis view, the defect was between the 12 o’clock and 11 o’clock positions. The gradient was steady at 44mmHg over three days. The course of the VSR was serpiginous and irregular. His apical IVS was dyskinetic and aneurysmal. His EPSS was 17cm.
The procedure was performed under general anaesthesia and trans-thoracic echocardiography (TTE) guidance. Intravascular sheaths were introduced into the right femoral vein (RFV),( 6F ) the right femoral artery (RFA) (7F ) and the right internal jugular vein (RIJV).(6F )
The internal jugular vein puncture was done in the standard way.Using a 6F Pigtail catheter, an LV angiogram was done that showed dye entering the right ventricle (RV) from the left ventricle (LV) through a single defect in the apical septum (Figure 1). Defect size was not measured using the LV angiogram, and it was decided to proceed further considering the size measured with the TTE. Therefore, we decided to proceed with a-16 mm Amplatzer ASD occluder device.
After the injection, a 6F JR3.5 guide catheter was passed over a 260-cm Terumo wire and passed through the RFA sheath (Figure 2). The distal end of the Terumo wire was used to cross the VSR defect, and the guide catheter was crossed into the RV and then into the left pulmonary artery (LPA) (Figure 3).

Figure 2 (a)The device seated across the vsr seen by echo (after deployment of the device. (b)The procedure -in steps -The LV angiogram of the patient with ventricular septal rupture. This angiogram was not used for sizing. The echocardiographic image was used for sizing.
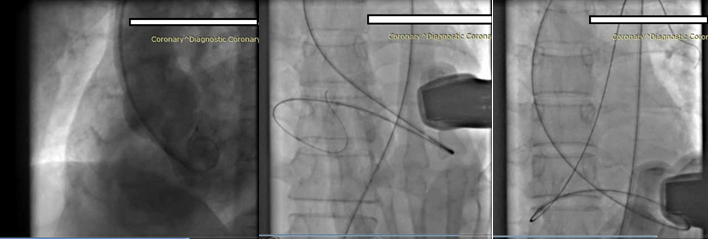
Figure 3 The VSR was crossed with a Terumo wire inside the right coronary artery catheter to prevent further tearing of the ventricular septum during manipulation.
Through the 6F sheath into the RIJV, a 15-cm Goose-neck snare wire was passed into the RV and then into the LPA. The Terumo wire was then snared. The distal tip of the Terumo wire was taken out of the RIJV sheath (Figure 4).
A 180° 10F PDA sheath was passed from the RIJV into the RV and across the defect into the LV. A 16-mm Amplatzer device (ASD device) was then deployed across the defect with the LV disk first and then the RV disk (Figure 5-8).

Figure 5 The PDA sheath was slowly introduced over the Terumo wire taking care that the right coronary guide catheter covered the wire at all times to prevent through and through cutting of the ventricular septum during the procedure.

Figure 7 The device was deployed first in the left ventricular disc and then the right ventricular disc.

Figure 8 Steps in deploying the device across the ventricular septum, gradually pushing the device out of the sheath.
The fluoroscopy time was 21 minutes, and the total dye used was 160ml of iohexol.
Post-deployment, the transthoracic echocardiogram showed minimal leakage across the defect. The patient tolerated the procedure well and was shifted to the ICCU for further management. He was electively ventilated for 24 hours.
The patient was put on the following drugs: 150mg/dayenteric-coated aspirin, 75mg/day clopidogrel, 40mg/day atorvastatin, 0.25mg/2 days digoxin , 3.125mg twice a day carvedilol and 40mg/day tab frusemide.
This report is not a sponsored study. No author has any relationship with the industry or conflicts of interest. Drs PNG and PV were in charge of the case, Dr S performed the echocardiograms, and Dr V retrieved the records and assisted with the case.
VSR device closure is now becoming more common. Since VSRs have such a devastating outcome, this terrible complication should be tackled.
If the VSR is smaller (as the largest VSD device available is 24mm) and the surgeons are reluctant to perform emergency surgery, the patient should be considered for device closure as early as possible. We used the Amplatzer ASD occluders. Assenza et al have opined that the ASD occluders from Amplatzer are suboptimal because they have a short waist. However, we did not have either the Star-flex device or the Cardio-seal device.6
The location of the ventricular septal rupture and ease of closure1
Ventricular septal ruptures can occur either in anterior wall myocardial infarctions or in patients with inferior wall myocardial infarction. It has been found that large ventricular septal ruptures at the apex without any rim are difficult to close with a device. In fact, a parachute device has been tried for such patients. However, small mid-muscular VSRs or apical VSRs with a rim can be closed with a device.
Inferior wall myocardial infarctions cause defects at the base of the heart at the junction of the septum and posterior walls.1 The ruptures can be simple or complex, called type 1 or type 2, respectively.1
Possible problems during device closure5
Patnaik and Barik listed the possible difficulties during device closure of a ventricular septal rupture. They attributed difficulties to the lack of knowledge of the procedure, tearing of the friable ventricular septum by the rigid sheath and the need for removal of the guidewire.
Is the device entangled in the mitral valvular apparatus or tricuspid valve apparatus? 8
If the device gets entangled in the mitral valve apparatus, the heart suddenly slows and the ejection fraction comes down. The device should be withdrawn into the sheath and should be redeployed.
Management after device closure
The patient should be put on drugs to unload the left ventricle and support the right ventricle. ( noradrenaline, milrinone or levosimendan. )
Evaluation after device closure:
If the patient has haemolysis or renal failure, one possibility is that there is a large residual shunt. A small or trivial residual shunt would unload the left ventricle.
The most common finding was sudden passage of deep red urine the day after the closure.Acute haemolysis also presented with a sudden drop in haemoglobin, a sudden increase in bilirubin or finding fragmented RBCs in the peripheral smear and fresh RBCs in the urine. These findings usually led to renal failure and would be ameliorated only by total closure of the residual defect.
We are sharing our experience because we were really helped by the reports in literature.4-6Other data with the Amplatzer and the Life Tech devices for VSRs are detailed in the following articles.12-16 Some studies have reported a 5 year survival after device closure.
In a systematic review, Theile and others reported that device closure should be done as early as possible.13 Furthermore, authors have even attempted a meta-analysis of post myocardial infarction VSR closure and found the survival to hospital discharge was 65% with a mortality of 37% at 19 months.14
Attia R and Blauth C. have summarized all of the recent series where post-myocardial infarction ventricular ruptures were treated by transcatheter closure.15 They concluded that patients with VSR sizes more than 1.5 cm usually ended up with embolized occluders. Therefore, they are better treated by surgery.
Holzer and Hijazi had a procedural success rate of 89% with a 30-day mortality of 28% and a long-term mortality of 61% (through 332 days).16 Therefore, they recommended that post-myocardial infarction VSRs should ideally be closed by the percutaneous route after 3.5 weeks of the index myocardial infarction when the size of the VSR is less than 1.5cm.15 However, many patients cannot survive that long.
Can VSR closure wait?
The ACC guidelines recommend closure as early as possible. We also believe this is the ideal strategy and the more the wait the more friable the tissues (our unpublished experience with two more cases of device closure).
We had analysed our data over a few years to predict which patients would survive 14 days after development of a VSR.( No device closure was attempted in these patients ) We found that VSRs with high gradients and smaller size live longer, and we have one such survivor (more than 2 years) and another late survivor (from 2004, more than 12 years). These are anecdotal cases. Generally, most of the VSRs die within 2 weeks of diagnosis if left alone, and they die in spite of surgery (only 4/7 survived to one month in our centre.17 The timing and the mode of treatment of this condition is still in conflict in 2019.18-22
Device closure should be attempted by all cardiologists, akin to the call to all cardiologists to perform CTO interventions in those patients who have a J CTO score below 2.
Device closure of a ventricular septal rupture is feasible. It should be performed as soon as possible in a patient who is unstable (increasing VSR size) increasing urea, and Serum creatinine or performed after 3 weeks in a stable patient. Transthoracic echocardiography and the trans jugular route for device deployment yield good results. Using a right coronary guide catheter to encase the Terumo wire at all stages prevents tearing of the ventricular septal defect during the procedure. Atrial septal defect devices can be used if the adequate sized ventricular septal device is not available.
The author declares no conflict of interest.
None.
None.

©2021 Gupta, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.