MOJ
eISSN: 2381-179X


Case Report Volume 6 Issue 3
Department of Critical care, Pulmonology and Sleep Medicine, India
Correspondence: Mani RK, CEO and Chairman, Department of Critical care, Pulmonology, and Sleep Medicine Nayati Medicity, India
Received: March 15, 2017 | Published: March 21, 2017
Citation: Mani RK, Mishra V, Sarma U, et al. Pitfalls in the ventilator management of myasthenia gravis, autonomic instability and anticholinesterase-induced airway resistance. MOJ Clin Med Case Rep . 2017;6(3):66-69. DOI: 10.15406/mojcr.2017.06.00161
A 31years woman, under treatment for Myaesthenia gravis and no past history of underlying respiratory disease, was admitted with breathlessness, diplopia and ptosis. On evaluation she was found to have a thymoma. Soon after admission she developed a severe Myasthenic crisis (MC) with respiratory failure requiring steroids, immunosuppressive, mechanical ventilation and Plasmapheresis. She also underwent thymectomy after stabilisation. Her postoperative ventilatory management was difficult, prolonged and complicated by autonomic instability, poor respiratory muscle function, and anticholinesterase-induced increase in airway reactivity. High airway pressures, airway resistance, and air trapping led to bilateral pneumothoraces requiring intercostal drainage. The airway reactivity and resistance settled after discontinuing pyridostigmine and instituting inhaled Ipratropium bromide, an anticholinergic agent. Pneumothoraces resolved rapidly and the patient could be weaned successfully via a tracheostomy. Autonomic instability also resolved with resolution of the stormy phase of illness, airway pressures, and liberation from ventilator. At outpatient follow up weeks later, airway reactivity to pyridostigmine was demonstrable with spirometry. Existing literature on the potential complications of anticholinesterase therapy during mechanical ventilation for Myasthenic crisis is sparse.
Keywords: myaesthenia gravis, myaesthenic crisis, airway resistance, pyridostigmine, mechanical ventilation, pneumothorax, thymectomy
MG, myaesthenia gravis; MC, myaesthenic crisis; Ach, acetylcholine; NIV, non invasive ventilation; PEFR, peak expiratory flow rate; AchRab, acetylycholine receptor antibody; ACMV, assist control volume targeted ventilation; D, day of hospitalization; RrS, airway resistance on ventilator in cmH20/L/sec; P Plat in cmH20; PYR, Pyridostigmine, Neo, neostigmine; PP, plasmapheresis; ET, endotracheal intubation; ICD, intercostal tube drainage; PNX, pneumothorax; Off Vent, off ventilator;Trach, tracheostomy; Decan, tracheostomy tube decanulation; MAP, mean arterial pressure; HR , heart rate
Ventilatory management of myasthenia gravis (MG) and myathenic crisis (MC) is often associated with weaning difficulties leading to significant morbidity and mortality. Severe autonomic instability manifesting as wide swings in heart rate and blood pressure, is only rarely reported.
In the above scenario, anti cholinesterase therapy used to treat myasthenia, may also prove to be counterproductive. Increased airway resistance due to anticholinergic therapy in ventilated patients of MG is not widely reported in the literature and can be missed unless monitored. The case described here is important as it highlights the pitfalls in ventilator management of MG. Attention to airway resistance and relating it to anticholinesterase therapy led to its timely discontinuation. Autonomic instability may be difficult to manage and is rarely described in detail.
A 31year old woman presented with a 2month history of worsening breathlessness, persistent fatigability and drooping of eyelids. This was against a background of intermittent eye symptoms and muscle weakness over 2years. MG was established on the basis of clinical, electromyographic and high anticholinesterase receptor antibody (AchRAb) levels (17.85nmol/dl). She had shown partial improvement on pyridostigmine and prednisolone but since 2months she had stopped all treatment. This was followed by persistent ocular symptoms, chest discomfort, dyspnoea of effort and orthopnoea, for which she presented to the outpatient department of the hospital. She had no previous history suggestive of underlying bronchial asthma or airway disease.
On Examination she was fully conscious , mildly tachypneic with a heart rate of 110/min, blood pressure 130/80 mmhg, and pulse oxymetry of 95 % on room air in the sitting position, near-complete ptosis ( Rt.>Lt), chest clear on auscultation, no respiratory paradox but was orthopnoeic , other systems were normal. A chest X-ray showed a mass on the right side extending from the root of the neck to the paracardiac region. computerized tomography (CT) chest revealed a superior mediastinal mass extending low into the right thorax suggestive of thymoma. Free T3, Free T4 and TSH were within normal range. Other routine baseline investigations were within normal limits. A 2D echo did not reveal any abnormality. She was admitted to the hospital and started on oral Pyridostigmine and prednisolone 10mg once daily. Dose titration of pyridostigmine was done and later intravenous neostigmine was added as respiratory symptoms remained uncontrolled. Intravenous glycopyrolate was given along with neostigmine to prevent the muscarinic effects.
On the 3rd hospital day, the patient was shifted to the intensive care unit for worsening dyspnoea. Non invasive ventilatory support was instituted. The peak expiratory flow rate was low for her height (156cm) at 130L/sec. She also had vertical diplopia, bilateral ptosis and facial weakness consistent with the diagnosis of a myasthenic crisis (MC). For the MC, oral prednisolone 10mg once daily (OD) and azathioprine 50mg OD were initiated. The single breath count was 16 to 26. Thymectomy was planned after stabilizing the patient. However, there was only partial relief leading to the dose of pyridostigmine being escalated to 15mg 4th hourly and prednisolone to 40mg OD. With worsening respiratory status plasmapheresis was started on the 7th hospital day. She received an exchange of 3liters which was well tolerated. AchRAb titer was found to be high at 17.85nmol/L (negative value<0.5nmol/L). On the 8thday, she required intubation of trachea and initiation of mechanical ventilator (MV) support. A low grade fever ensued and on the appearance of a right lower infiltrate she was started on intravenous clindamycin 900mg thrice daily suspecting aspiration pneumonia. Feeding was continued through nasogastric tube. Two to 3days after the onset of MC the patient also started to have wide fluctuations in blood pressure and heart rate (Figure1) (Figure 2). These fluctuations were both spontaneous and coinciding with anxiety, pain, discomfort and ICU procedures especially endotracheal suctioning which is highly suggestive of autonomic instability. This was managed with targeted sedation and analgesia protocols, and judicious mobilisation.
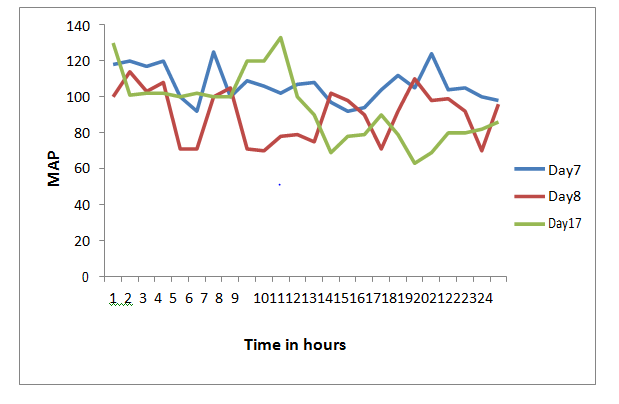
Figure 1 Graph of MAP (y axis) and Time of the day in 24hour clock. Shows marked fluctuation in MAP during the course of illness as depicted by hourly MAP readings on day 7, Day 8, and Day 17 of hospitalization. This fluctuation occurred spontaneously, accentuated with anxiety, pain, and ICU procedures including endotracheal suction. This suggests marked Autonomic instability. Abbreviations: MAP, mean arterial pressure
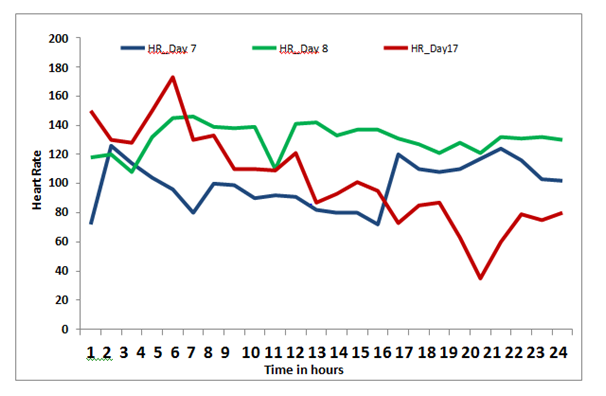
Figure 2 Graph of Heart rate (HR) (y axis) and Time of the day in 24hour clock. Shows marked fluctuation in HR during the course of illness as depicted by hourly HR readings on day 7, Day 8, and Day 17 of hospitalization. This fluctuation occurred spontaneously, accentuated with anxiety, pain, and ICU procedures including endotracheal suction. This suggests marked Autonomic instability.
A second cycle of Plasmapheresis was administered .Thymectomy was performed after the 3rd cycle of plasmapheresis (day13) Intra operative period was uneventful. A large lobulated thymic mass of heterogenous consistency was found adherent to the middle lobe of the right lung near the hilum. Histopathology of the specimen revealed histological type (WHO) of B2 Thymoma with a pathological staging (Modified Masaoka) stage 2a.
Prednisolone and Azathioprine were reinstituted after the immediate, uncomplicated post operative period. Following surgery, she was given three cycles of plasmapheresis on alternate days. She was switched to pressure support ventilation with an inspiratory pressure of 20 Cm H2O. On the 16th day her respiratory condition deteriorated requiring assist-control volume targeted mode (ACMV). Respiratory mechanics revealed an alarming increase in respiratory system resistance ( Rrs) rising rapidly from 11 to 25cm of H2O/L/sec and plateau pressure from 17 to 35cm H2O , unresponsive to nebulized beta agonist salbutamol and the anticholinergic ipratropium bromide combination. A chest X-ray showed bilateral pneumothorax.
Bilateral intercostal drainage tubes (ICD) were placed despite which Rrs remained high. Suspecting cholinergic agent induced bronchoconstriction, cholinergic drugs were reduced, then stopped and the bronchodilator medication switched to ipratropium alone. This was followed by rapid correction of lung mechanics. She was tracheostomised on the 22nd day due to weaning failure. Immunomodulation was enhanced to prednisolone 60mg OD and azathioprine 50mg twice daily.
Subsequent clinical course showed steady all round improvement with stable pulse, blood pressure and airway resistance. Other medications included cefepime infusion for suspected intercurrent blood stream infection, fentanyl infusion in the early postoperative period and thromboprophylaxis. The ICDs were removed after 24hours. Reintroduction of pyridostigmine in modest doses resulted in immediate increase of airway secretions, rising Rrs and respiratory distress necessitating its withdrawal. Complete weaning from MV could be accomplished on the 25th hospital day. Decannulation of tracheostomy was done on the 28th day. She remained asymptomatic with no ptosis, diplopia or fatigability of muscles. At discharge on the 34th day she was ambulant and free of symptoms (Table 1).
|
D 7 |
D 8 |
D 9 |
D 11 |
D 15 |
D 16 |
D 17 |
D 19 |
D 21 |
D 22 |
D 28 |
D 31 |
PYR |
480 |
Nil |
nil |
180 |
240 |
240 |
240 |
120 |
nil |
nil |
nil |
15 |
NEO |
12 |
3 |
nil |
nil |
7.5 |
10 |
nil |
nil |
nil |
nil |
nil |
Nil |
RrS |
10 |
11 |
15 |
25 |
17 |
14 |
10 |
|||||
P plat |
17 |
19 |
21 |
35 |
22 |
18 |
18 |
|||||
Events |
PP |
ET |
PP |
PP |
PP |
|
Pnx ICD |
ICD removed |
Trach |
|
Off vent |
decn |
Table 1 Trends of anticholinesterase doses(mg/day) and airway resistance (Rrs) during the course of treatment
This table shows increasing Rrs with escalating dose of anticholinesterase which was maximum on day 16(240 mg of pyridostigmine and 10mg of neostigmine) leading to severe increase in Rrs, P plat on day 17 that caused B/L pneumothorax. Despite B/L ICD placement R and P Plat continued to be high. Stopping all anti cholinesterase treatment was followed by stabilisation of ventilatory parameters.
Abbreviation: PYR, pyridostigmine; NEO, neostigmine; Rrs, airway resistance on ventilator, Pplat, platue pressure; PP, plasmapharesis; ET, endotracheal intubation; Pnx ICD, pnuemothorax with insertion of ICD done; Off Vent, off ventilator; Decn, tracheostomy tube decanulation; ICD, intercostal tube
MG is a relatively rare autoimmune disorder in which antibodies are generated against acetylcholine nicotinic postsynaptic receptors at the neuromuscular junction of skeletal muscles. The patient described above presented with a characteristic pattern of muscle fatigue with effort, and recovery after a period of rest. MC is a complication of MG characterized chiefly by rapid worsening of muscle weakness, resulting in respiratory failure that may require intubation and MV.
In the present case MC was diagnosed on the basis of escalating dose of anti cholinesterase therapy culminating in ventilatory assistance, as per current consensus.1–4 Respiratory infection, stress of surgery, trauma or emotions have been identified as triggers of MC. Plasmapheresis was aimed at reducing the circulating antibodies to acetyl choline receptors. The Antibody level remained high despite significant clinical improvement after 5 cycles. Use of plasmapheresis in the treatment modality of MG was reported as early as 1976 by pinching et al.,5 Plasmapheresis is known to improve respiratory function when compared with pyridostigmine and is found to be useful in ventilator dependency in a non randomized trial of nine patients.6 The use of plasmapheresis to control MC or in preoperative preparation of thymectomy remains inadequately studied.7 In our case clinical course was complicated by autonomic instability, a rare but recognized manifestation of MG. There are reports of autonomic dysfunction in MG, particularly in association with thymoma.8 Shukla et al.,9 in a prospective study of 64patients with MG observed a significant rise in heart rate and blood pressure as compared to a control group when tested for autonomic dysfunction by orthostasis and isometric handgrip tests.9 There was no significant difference in parasympathetic function. Peric et al.,10 found similar results in 21 patients with the standard cardiovascular reflex test. In our case autonomic instability was severe and prolonged complicating the management of the patient.11–13
As we continued pyridostigmine in the post operative period the patient on MV developed increased airway resistance leading to bilateral pneumothoraces. This phenomenon is attributable to cholinergic- associated excessive secretions and bronchoconstriction. Shale and colleagues14 in a survey of 21patients with MG on oral Pyridostigmine 60 to 120mg, found air flow limitation in 8 patients . In a double blind study, six patients undergoing pulmonary function testing while continuing pyridostigmine with or without ipratropium bromide. Increase in Rrs associated with pyridostigmine was reversed completely with inhaled ipratropium bromide. Liget et al.,15 also made similar observations in three cases of MG with chronic obstructive pulmonary disease who were on oral pyridostigmine therapy, two of whom on moderately high dose (>600mg/day).
Respiratory function worsened with anticholinesterase therapy that was reversed by inhaled ipratropium bromide attributable to either bronchodilation or reduction of secretions. Our case illustrated similar effects of cholinergic agents albeit in the setting of mechanical ventilation, with potentially more serious consequences.
The dose response relationship of pyridostigmine with the respiratory system has been studied in relation to the plasma cholinesterase activity. Neostigmine in doses of 0.5mg/kg, 2mg/kg and 5mg/kg was injected intravenously to anaesthetized dogs. Hemodynamic parameters and pulmonary functions were measured. Airway resistance increased 10-fold as neostigmine dose rose from 2mg/kg to 5mg/kg.16 However, a single dose of neostigmine of 0.015mg/kg as used in the reversal of neuromuscular blockade with or without atropine 0.0075mg/kg was found not to have any statistically significant effect on airway resistance.17
In the case described, we discontinued the cholinergic agents till the patient could be liberated from MV as was done in earlier studies.18,19 Subsequently in outpatient follow up, reinitiation of small doses of pyridostigmine again resulted in bronchial hyper responsiveness as demonstrated by spirometry. She was therefore managed successfully on immunomodulators alone. Larger controlled studies are needed to assess the impact of cholinergic agents on airway resistance and clinical outcomes in MG with and without mechanical ventilation.
None.
The author declares no conflict of interest.

©2017 Mani, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.