eISSN: 2574-9927


The influence of the weld metal composition on the stress corrosion cracking (SCC) susceptibility of dissimilar weldments between AISI 316 and 2304 duplex steels was investigated by constant load SCC tests and metallographic examination. Two electrodes (E316L and ER 2209) were used to produce fusion zones of different chemical compositions. The SCC test results showed that the region with lower SCC resistance was the interface between the fusion zone and heat affected zone (HAZ) on the 316 base metal side of the weldments for both filler metals tested. The SCC results also showed that the weldments with 2209 duplex steel filler metal were less resistant to SC cracking when compared to the weldments with 316L filler metal. This lower SC resistance of the dissimilar joint with 2209 duplex steel filler metal may be related to the simultaneous presence of continuous vermicular delta ferrite and sigma phase in the HAZ on the 316 base metal side. This favored the nucleation and propagation of SC cracks, once the presence of secondary phases reduces corrosion resistance and toughness of HAZ, and delta ferrite acts as an anode and it is preferably attacked in relation to austenite in the 316 stainless steels.
Keywords: stress corrosion cracking, dissimilar weldment, stainless steels, microstructure
A considerable number of components used in the oil and gas industries such as chemical tanks, ducts, pump valves and shells, pipelines, etc. and that, are frequently in contact with some sort of corrosive environment, are manufactured of austenitic stainless steels types 316, 316L, 316LN and, to a lesser extent, 317LN due to their good mechanical and corrosion resistance.1,2
However, today the movement towards the use of duplex stainless steels, of austenitic-ferritic structure, in replacement to austenitic stainless steels, is increasing considerably.1 This is attributed to the fact that the duplex stainless steels have much higher stress corrosion cracking (SCC) resistance than austenitic stainless steels.
Among several manufacturing process used to repair and fabricate the components mentioned above, welding is the main process used and it may contribute significantly to the stress corrosion cracking. SCC is a local corrosion process, which is characterized by the slow nucleation and propagation of cracks on the material under the simultaneous action of tensile stresses and a specific corrosive environment.3 In general, the nucleation of stress corrosion cracks occurs at stress levels below the yield stress of the material and typically below the design tension and fatigue limit of a structural component.4,5
According to literature,5,6 during dissimilar welding involving particularly duplex stainless steels, certain problems can arise during manufacturing of several components. In these steels, during welding, the heat affected zone (HAZ) often experiences critical temperatures that are sufficient to produce almost fully ferrite in this region. As cooling starts, the formed ferrite undergoes a solid‒state transformation to austenite from the grain boundary and then continues in the ferrite grains. The extent of ferrite to austenite transformation depends on the base metal and filler metal composition and welding parameters. Considering that in the welding process the cooling rate is high, significant ferrite content can remain in the HAZ and precipitation of intermetallic phases such as sigma (σ), chi (χ) and laves phase can occur at locations that have reached temperatures above 900°C with suppression of ferrite-to austenite phase transformation. This can significantly reduce mechanical properties, pitting and stress corrosion cracking resistance of the stainless steel dissimilar weldment.7,8
Prior works,9‒11 have reported that, in dissimilar welding involving austenitic stainless steels and other type of stainless steel with quite different contents of Cr, Ni and Mo, the delta ferrite and sigma phase content present in the weld metal and HAZ have a significant influence on the susceptibility to stress corrosion cracking of the weldment once these phases are electrochemically more active than austenite phase, resulting in preferential corrosion attack of the weld regions enriched to these phases, when exposed to aggressive environment.
It is worthwhile mentioning that sigma phase is formed mainly of iron and chromium but it may contain small contents of molybdenum as well. As a result, variations in the chromium and molybdenum contents in the filler metal and base metal chemistry modify the kinetic reactions and modify significantly the elemental partitioning and, consequently, the volume fraction of delta ferrite and sigma phase. According to literature,6 when the base metal and/or filler metal contain levels of chromium above 20% and molybdenum in small amount, sigma phase precipitation is very prone to occur in the HAZ of the dissimilar stainless steel weldment.
In attempt to mitigate the problems mentioned above and improve the stress corrosion cracking resistance of this dissimilar weldment, some studies of techniques and consumables for welding between duplex and austenitic stainless have been conducted. Basically, these studies consist to verify the influence of the welding thermal cycle (which is directly dependent of the welding parameters) and filler metal composition on the delta ferrite content and on elemental partitioning in weld metal and HAZ in order to reduce the susceptibility to stress corrosion cracking.
Therefore, this study aims to evaluate the influence of two different filler metal (E316L and E2209) on the microstructure and on the SCC resistance of dissimilar stainless steels weldments between 316 austenitic stainless steel and 2304 duplex stainless steel in an environment containing boiling chloride. The correct selection of the filler metal may be an efficient practice to minimize the occurrence of SCC in repair welds of industrial equipments.
The base materials used in this work were AISI 316 austenitic stainless steel and 2304 duplex stainless sheets of 4mm thickness. The filler metals used were AWS E316L and AWS 2209 duplex stainless steel solid wires with a 1.2mm diameter. These filler metals are usually used in welds of stainless steel components in petroleum and sugar and alcohol industries. Table 1 presents the chemical compositions of the base and filler metals used.
|
C |
Mn |
Si |
Cr |
Ni |
Mo |
N |
AISI 316 |
0,032 |
1,367 |
0,49 |
16,2 |
9,2 |
1,993 |
- |
UNS 32304 |
0,02 |
1,356 |
- |
22,23 |
3,61 |
0,31 |
1090 ppm |
ER2209 |
0,03 |
1,7 |
0,5 |
22,5 |
8,5 |
3,3 |
- |
E316L-16 |
0,022 |
0,69 |
0,96 |
18,51 |
11,79 |
2,70 |
0,0580 |
Table 1 Chemical composition (% weight) of the base and filler metals
The manual welding was carried in two passes deposition on flat position on V‒chamfered 65×300mm sheets, using GMAW process. The welding parameters used in this work are shown in Table 2.
Gas flow |
Shielding gas |
Voltage |
Current |
Travel speed |
Wire feed speed |
(l/min) |
(%) |
(V) |
(A) |
(m/min) |
(m/min) |
14 |
Argon-2 % O2 |
19 |
120 |
0.3 |
4 |
Table 2 Welding conditions used in this study
The same welding parameters were used for both filler metals. These values were selected within the range usually used by the weld manufacturer of components subject to stress corrosion cracking.
SCC testing
A commonly service condition found in the petrochemical industry is that where the components are subjected simultaneously to a temperature range of 140 to 150°C and aerated corrosive environment contained chlorides. In order to reproduce this service condition in the SCC tests is important to point out that the boiling chloride media temperature is directly related with the chloride concentration. In this work, therefore, to evaluate the susceptibility to SCC of the weldments, were used an aerated boiling 43% MgCl2 test solution at 145±3°C and a constant‒load SCC apparatus. 4mm thick smooth rectangular tensile specimens (Figure 1) were used for the SCC tests. The geometry and preparation of the specimens was in accordance with ASTM G58,12 and ASTM E8,13 standards.
Specimens and test solution were confined in a glass cylinder which was coupled to a reflow condenser so that all measurements were monitored continuously under closed‒circuit conditions. Tests in three specimens of each filler metal were conducted at a tensile load correspondent to the nominal stress level of 250MPa; which was associated with SCC dominated failure in this type of environment for stainless steels weld metals.14,15 Time‒to‒failure was the SCC parameter evaluated.
Microstructural characterization
Rectangular specimens were sectioned transverse to the surface of the specimens before and after SCC tests, then polished and etched electrolytically in 10% oxalic acid to reveal the microstructure of the weldments. This etching reveals the ferrite as a dark phase in the bright austenite matrix in the weld metal. Microstructural examinations of the specimens were carried out using standard optical microscopy coupled to an image analysis system, scanning electron microscopy (SEM) and XRD technique.
As mentioned in the experimental procedure, the necessary time for occurrence of the complete fracture was the parameter adopted to evaluate the susceptibility to the stress corrosion cracking of the weldments. Table 3 shows the average time and temperature values of the test for both filler metals.
Weld metal |
Temperature (ºC) |
Time-to-fracture (h) |
|||
Tf 1 |
Tf 2 |
Tf 3 |
Tf Average |
||
ER 2209 |
145± 1 |
77 |
85 |
90 |
84 |
E316L-16 |
145 ± 1 |
112 |
100 |
94 |
102 |
Table 3 SCC tests results
The SCC results showed that the welds carried out using filler metal AWS ER316L‒16 were more resistant to cracking than those carried out using the filler metal AWS ER2209. On the other hand, SCC results also showed that the specimen fractures, for both filler metals, occurred predominantly in the HAZ on the 316 austenitic metal base sides (Figure 2), that is, the HAZ of the 316 steel was the most susceptible region to stress corrosion cracking.
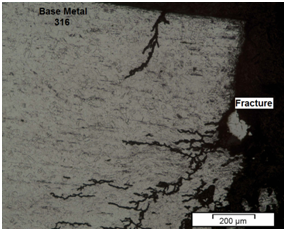
Figure 2 Microstructure of dissimilar weldment with E316L weld metal showing stress corrosion cracks in the HAZ/FZ interface.
The higher susceptibility to SCC in this region may be attributed mainly to the presence of a continuous delta ferrite network in the fusion zone and in the HAZ of these steels (Figure 3) (Figure 4). According to,16 the development of SC cracks along to the ferrite boundaries is because the delta ferrite acts as anode (more electrochemically active), protecting the austenite, which acts as cathode. Additionally, the ferrite phase (anode) is attacked more easily by the environment than the austenitic phase (cathode), particularly due to the segregation of impurities in it.
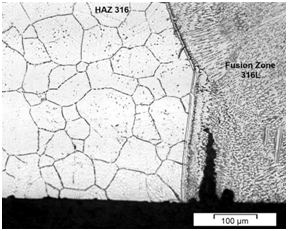
Figure 3 Microstructure of dissimilar weldment with E316L weld metal showing a stress corrosion crack in the fusion zone.
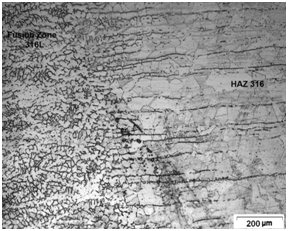
Figure 4 Microstructure showing the presence of delta ferrite network in the fusion zone and aligned delta ferrite in HAZ of the dissimilar weldment with E316L weld metal.
It is also worthwhile to note that in the dissimilar weldment using 316L stainless steel filler metal, it was observed cracks in the HAZ on the 2304 duplex metal base side, as can be seen in Figure 5. However, SC tests and microstructural evaluation indicated that propagation of SC cracks in this region were slower. This may be attributed to the slight presence of deleterious phases (sigma phase and Chi phase) and the absence of delta ferrite in this region. According to literature,2,17 the presence of sigma phase, not only reduces corrosion resistance, but also embritles considerably the HAZ, decreasing its toughness; which tends to facilitate the nucleation and propagation of stress corrosion cracks.
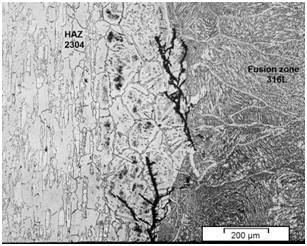
Figure 5 Microstructure of dissimilar weldment with E316L weld metal showing the presence of corrosion stress cracks in the HAZ/FZ interface on the 2304 duplex steel base metal side.
The high level of Cr and the low level of Mo and Ni present on 2304 duplex steel combined with the higher levels of Ni and Mo and lower levels of chromium in the 316L filler metal may have contributed to a more uniform and balanced partition of these elements during the weld cooling, thus avoiding the formation of significant amount of sigma phase.
However, it is important to investigate the reason why joints welded with 2209 duplex steel filler metal presented lower SCC resistance (smaller fracture time), once for these joints, the most susceptible region was the HAZ on the 316 base metal side as well. The microstructural evaluations of type 316 base metal region for these joints and DRX results show that the lower SCC resistance of this joint is because of the combined presence of delta ferrite networks (Figure 6) (Figure 7) and sigma phase ( Figure 8) (Figure 9) in the HAZ of 316 base metal. EDS Microanalyses in the black precipitates shown in Figure 8 indicated that they are widely enriched of Fe (75.4%) and Cr (24.27%) and presented a certain amount of Mo (0.35%), which are the main forming elements of sigma phase.
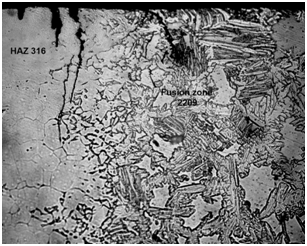
Figure 6 Microstructure of dissimilar weldment with E 2209 weld metal showing corrosion stress cracks in the HAZ on the 316 steel base metal side. Note the presence of delta ferrite network which facilitated the crack propagation.
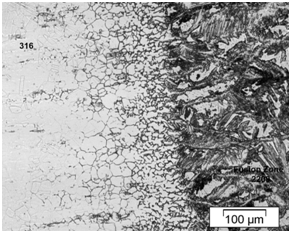
Figure 7 Microstructure of dissimilar weldment with E 2209 weld metal before SCC tests showing a presence of delta ferrite networks.
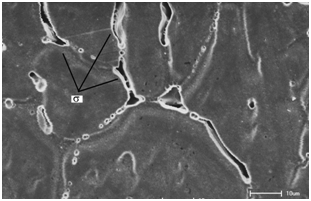
Figure 8 Microstructure of the HAZ of dissimilar weldment with 316L weld metal showing precipitates in the austenite/ferrite interface (sigma phase).
Therefore, from these figures, it is reasonable to affirm that the lower corrosion resistance and toughness of this region due to the presence of sigma phase accelerated the crack nucleation process, and the presence of delta ferrite networks facilitated the crack propagation; which lead to smaller fracture time for this weldment if compared to the weldment using 316L stainless steel filler metal.
It is important to emphasize that in the case of the weldment using 2209 duplex stainless steel filler metal, the chemical composition of both type 316 base metal and, mainly 2209 filler metal facilitates the formation of delta ferrite and of sigma phase in the HAZ of 316 base metal, as shown in the figures. This may be due to the higher levels of chromium and molybdenum of 2209 steel in comparison to the levels observed in the 316 stainless steel base metal. Due to weld dilution of 25% of the MIG process and subsequent weld cooling, part of the filler metal chromium and molybdenum tend to migrate to the 316 base metal HAZ once the concentration gradient of these elements is reasonably high. According to the literature,18‒20 these elements strongly influence the delta ferrite and sigma phase formation. Moreover, in general, the sigma phase arises from the delta ferrite, where the elements Cr and Mo are concentrated.
Figure 10 shows that there was no occurrence of SC cracks in the HAZ of the dissimilar weldment using 2209 duplex filler metal, on the base metal 2304 side.
The dissimilar weldment region which presented higher susceptibility to SCC, for both filler metals, was the HAZ of AWS 316 austenitic stainless steel. This was because these steels form semi‒continuous delta ferrite networks in the HAZ and fusion zones, which facilitate the propagation of SC cracks through the boundary of ferrite grains.
The dissimilar weldment using type 316L stainless steel filler metal presented more resistance to SCC than the dissimilar joint using 2209 duplex stainless steel. This may be attributed to the simultaneous presence of a higher content of continuous delta ferrite (network morphology) and sigma phase in the HAZ of 316 base metal when duplex steel filler metal was used.
The presence of the higher content of delta ferrite in the HAZ of the 316 base metal when duplex filler metal was used may be attributed to the higher migration of chromium from the fusion zone to HAZ of this steel during solidification of the weld metal once the chromium content of the filler metal was considerably higher.
None.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.