eISSN: 2574-9927


Research Article Volume 3 Issue 4
Departamento de Quimica, Universidad Nacional del Sur, Argentina
Correspondence: Marisa A Frechero, INQUISUR-Departamento de Quimica, Universidad Nacional del Sur (UNS), Av. Alem 1253, CP 8000, Bahia Blanca, Argentina
Received: June 27, 2019 | Published: July 17, 2019
Citation: García LAH, Molina MC, Zoratti M, et al. Ion-polaron interaction in modified tellurite glasses. Material Sci & Eng. 2019;3(4):113-116. DOI: 10.15406/mseij.2019.03.00100
Glasses formed by different oxides are of uppermost importance because they are developed for many applications in electronics, optics, biology, etc. Oxide glasses are built by a three-dimensional network of corner connected oxygen polyhedral that can be modified by the inclusion of other oxides such as transition metal oxides. In this work, the polaron conductivity due to the presence of transition metal oxides in tellurite glasses is analyzed in systems containing alkaline (AO) and alkaline earth oxides (AEO). It is explained how the presence of metal cations (AO or AEO) affects the displacement of the polaron through the network due to the ion-polaron interaction.
Keywords: glasses, oxide creates, polaron, cations, polarization, electron hops, tellurite glasses
AEO, alkaline earth oxides; XRD, X-ray diffraction analysis; Ea, activation energies
Glasses formed by a mix of oxides are interesting for many technological applications. Their physical properties change according to the constituent oxides. Every oxide creates a three-dimensional network built by corner connected oxygen polyhedral, with different coordination number. The oxides can be either glass formers or glass modifiers, granting the final product specific characteristics. Thus, it is of utmost importance which oxides to incorporate to the original mix. In this work, we analyze the polaron conductivity in the presence of large alkaline cation concentrations. We studied oxide glasses formed by TeO2 modified by the incorporation of transition metal oxides: V2O5, Cu2O, and MoO3. Additionally, such tellurite glasses contain Na2O or MgO. Holstein1 proposed in 1959 for materials with low charge carrier mobility (<0.1cm/V.s) that an electron trapped in the lattice would not be able to move unless the lattice could move together with it. Such proposal gave birth to the polaron concept. The explanation is that the charge carrier (the electron) induces a dipole moment on its neighboring and both move together, i.e. the polaron. Therefore, polaron conductivity involves the displacement of polaron in a material. When the material is a glass the charge carrier and the distortion in its surrounding move through the glassy matrix. For that reason, in this work we study how the presence of alkaline and alkaline earth cations affects the polaron transportation in the tellurite glasses.
The glasses studied in this work have the general formula: xMiOj (1-x)[0.5V2O5 0.5MoO3] 2TeO2 (M=Mg, Na, Cu) and were prepared by a standard melt quenching technique starting from reagent grade chemicals. Appropriate amounts of the components were well mixed and placed in a platinum crucible and heated at 1073K in an electric furnace for half an hour, frequently shaking the crucible to ensure homogenization. The molten material was poured on a preheated aluminum plate forming isolated drops and finally annealed for 2hours at 450K. The amorphous character of the samples was tested by X-Ray Diffraction analysis (XRD) with a Bruker D8 Advance diffractometer in continuous scan mode with a copper anode and 45kV–30mA at room temperature in the 2θrange: 10°–60°. Electrical characterization of the samples Agilent 4284A analyzer within the range of frequencies from 20Hz to 1MHz as a function of temperature always 15K below its Tg to avoid sample structural changes. Density measurements were done following the Archimedean’s method using 2-propyl alcohol as secondary displacement medium.
The studied glassy systems are: (Table 1).
Glass name |
Glass formula |
MgVMoTe |
xMgO (1-x)[0.5V2O5 0.5MoO3] 2TeO2 |
CuVMoTe |
xCu2O (1-x)[0.5V2O5 0.5MoO3] 2TeO2 |
NaVMoTe |
xNa2O (1-x)[0.5V2O5 0.5MoO3] 2TeO2 |
Table 1 The studied glassy systems
Theoretical background
Considering that the polaron hopping theory2 was originally used to explain transport in doped or non-doped semiconductors where electrons are distributed in a hydrogen-type orbital according to the wave function: exp(−2αR), the electron is localized according to a landscape of potential values created by the modifier ion incorporated in the glassy matrix. A competition appears between the potential energy variation and the distance that the electron can hop. Therefore, the expression for the electron hopping rate (η) to another site at a distance R with carrier energy is: and η results proportional to the electrical conductivity (σ)2. The number of transition metal ions per unit volume in the glassy matrix gives the ion–ion average distance, which influences the electron hop. The factor exp (−2Rα ) in the hopping rate equation is the overlap integral and when it is accepted that the hopping mechanism is adiabatic, and the semiconducting behaviour is controlled by the activation energy. When the hopping mechanism is non-adiabatic (α≠0) the σdc is proportional to exp(−W/kT). Considering that in the polaronic transport theory there are two characteristic lengths: the average distance between sites (R) and the polaron radius (rp), an estimation of the localized polarization the electron hops from Mred+ to Moxid+ ion depends on both quantities. In our work, we compute (R) and (rp) from the impedance data and we analyse such values as function of the AO and AEO content in our tellurite glasses. Both lengths, R and rp, are calculated based on the glass composition and density applying the following equations:
(1)
(2)
Where N is the number of metal transition ions per molar volume (Table 2). Molar Volume [VMcm3], R [nm] and W [eV], values of W estimated from experimental dc conductivity data (slope in Figure 1).
The tellurite glasses studied here could have two possible electrical carriers: polarons and/or ions according to the content of the AO or AEO. Considering that vanadium oxide is present in the glassy matrices and they -the vanadium ions- appear in mixed oxidation states, i.e. Vreduced and Voxidized, the polaronic conductivity results from an electron transfer between those centers3‒6. As the electron travels within the matrix a distortion emerges, and they move together as a polaron and the dc conductivity in the system follows the equation:7
(3)
Where c and (1-c) are the occupied and available sites (Vreduced and Voxidized); R is the average distance between two adjacent V; υo is the phonon frequency; α is the tunneling factor; W is the activation energy; kB and T are the Boltzmann constant and the absolute temperature. Consequently, the conductivity temperature dependence is (Figure 2):
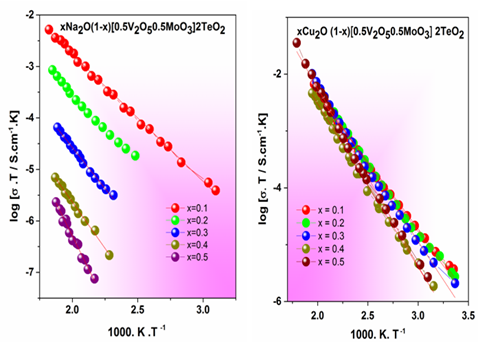
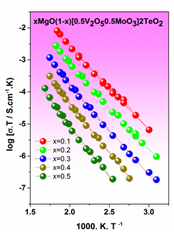
Figure 2 Temperature dependence of glasses dc conductivity: xNa2O (1- x) (0.5V2O50.5MoO3) 2TeO2; xCu2O (1- x) (0.5V2O50.5MoO3) 2TeO2 and xMgO (1- x) (0.5V2O50.5MoO3) 2TeO2.
(4)
The W in Eq. 4 is the potential barrier that the electron has to overcome due to the phonon vibrations between the Vred and the Voxid.8 From the results we learn that as the vanadium ions content diminishes, such potential barrier increases because of the increase in R.9,10 Therefore, the conductivity decreases. Previously it was demonstrated that only the vanadium ions are engaged in the process because the incorporation of molybdenum ions does not involve a higher energy barrier.11 As the V2O5 concentration decreases also the ratio Vred/Voxid is conditioned because it depends strongly on its redox surroundings.12,13 Figure 3 shows a conductivity diminution as the V2O5 content is replaced by MgO or Na2O; but almost any change is observed when Cu2O replaces V2O5. Even so, the reduction in the content of V2O5 causes a similar change on the Vred- Voxid average distance as we can see from R values in Table 2, but the replacement of V2O5 by Cu2O does not change the conductivity, it remains almost constant. Therefore, it is possible to assume that the polaron hopping is not affected by the mixed type of sites (weather it was V2O5 or Cu2O) and a unique path of reduced/oxidized sites is present for the polaron hopping (Figure 3). Further, it is interesting to notice that when MgO replaces V2O5 the conductivity diminishes because of the diminution of polaron concentration but also because of the potential landscape change due to the incorporation of the MgO in the glassy matrix. In addition, this change is stronger when Na2O is incorporated because the path is severely interrupted and the ionic conductivity due to free mobile Na+ also disturbs the polaron transport because the ion-polaron interaction appears. Therefore, the mixed conductivity explains the changes of the W values showed in Table 2. In 1996 Bazan et al.,14 confirmed the Kraevsky’s findings15 and explained the hypothesis of the interactions between polarons and mobile cations. Such interaction is the source of the anomaly observed in the conductivity behavior because the polarons are attracted by the opposite charge of the mobile cations. Then, a neutral pair moves, and it does not contribute to the electrical conductivity. The authors in that work explained the ion-polaron effect, the maxima in the activation energies (Ea) and the pre-exponential factors of the conductivity Arrhenius expression (σ0). The ion–polaron interaction causes the reduction of the electrical conductivity in mixed conductors due to the slower charges displacements caused by the Coulomb interactions. Such explanation allows for better understanding of the relationship between polaronic and ionic in the whole glass conductivity.[1] (Figure 4).
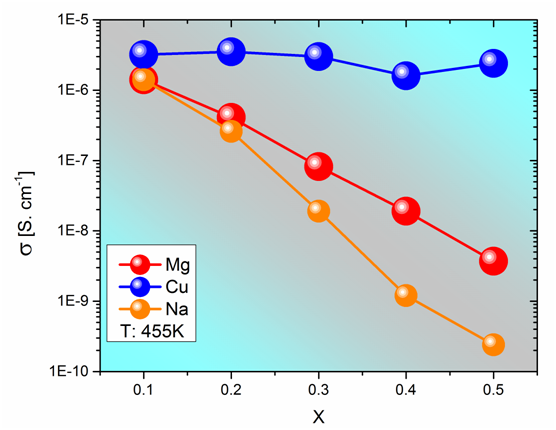
Figure 3 Isotherm of conductivity at around 180ºC as a function of modifier oxide content (x) of systems: xMgO (1- x) (0.5V2O50.5MoO3) 2TeO2; xCu2O (1- x) (0.5V2O5 0.5MoO3) 2TeO2 and xNa2O (1- x) (0.5V2O5 0.5MoO3) 2TeO2.
The electrical conductivity results plotted in Figure 3 can be understood through the pre-exponential factor of the Eq. 3. In such equation, the displacement of one electron from site to site between two sites with similar energy level is considered, the phonon scattering gives the equalization of two adjacent positions and the overlapping of the wave function is the factor exp(-2αR). Therefore, in the following equation:16
(5)
It is assumed that there are three fundamental factors: 1- [c. (1-c)] is the probability of having a donor and an acceptor site in an adjacent position; 2- [ )]: is the probability of two adjacent sites having the equivalent energy; and 3- [exp(-2αR)] is the probability of an electron to hop from site to site, i.e the tunneling factor. Therefore, from the conductivity results in Figure 3 & Figure 2 and the parameters in the Table 2 above, it is easy to understand computing , and in analogous manner for s0Cu/s0Mg. Considering that:
We conclude: and similarly that . This is notorious result. Such result is in good agreement with the Anderson theory (1958) which considered that a random small non-periodic potential affected the migration of an electron in a system.10
|
|
VM[cm3] |
R[nm] |
W [eV] |
|||||
x |
Mg |
Cu |
Na |
Mg |
Cu |
Na |
Mg |
Cu |
Na |
0.1 |
22.90 |
33.86 |
33.79 |
0.50 |
0.57 |
0.57 |
0.52 |
0.47 |
0.49 |
0.2 |
22.87 |
33.03 |
32.60 |
0.52 |
0.59 |
0.59 |
0.54 |
0.48 |
0.49 |
0.3 |
22.80 |
32.06 |
32.88 |
0.55 |
0.61 |
0.61 |
0.55 |
0.53 |
0.64 |
0.4 |
23.43 |
31.46 |
31.27 |
0.58 |
0.64 |
0.64 |
0.61 |
0.57 |
0.72 |
0.5 |
23.62 |
30.96 |
30.77 |
0.62 |
0.68 |
0.67 |
0.64 |
0.62 |
1.03 |
Table 2 Molar Volume [VMcm3], R [nm] and W [eV], values of W estimated from experimental dc conductivity data (slope in Figure 2)
The analysis made here reveals that the entity originated from the interaction between a charge carrier (electron) and the dipole moment induced on surrounding, so called polaron, causes the main conductivity phenomenon in a material. When the material is a tellurite glass, the charge carrier and its distortion in its surrounding are strongly affected by the presence of free mobile ions in the glassy matrix as Na-system and Cu-system show. Therefore, the presence of mobile cations affects the polaron through the change on the landscape and the model condition of the original theory developed by Anderson is not strictly satisfied. The existence of a not small perturbation given by mobile cations involves a strong effect on the non-periodic potential added to the formation of ion-polaron neutral entity in the studied modified tellurite glassy matrices.
I. L.A.H.G. and M.Z. are fellows of CONICET Argentina.
II. M.C. M. is fellow of ANPCyT Argentina
III. E. C. C. is Researcher Fellow of CIC PBA Argentina.
IV. S.T and M.A.F are Researcher Fellow of CONICET Argentina.
Authors declare that there is no conflict of interest.

©2019 García, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.