eISSN: 2574-9927


Research Article Volume 8 Issue 1
1Department of Nuclear Physics Technologies, State Institution, The Institute of Environmental Geochemistry of National Academy of Sciences of Ukraine, Ukraine
2Department of High Pressure Technologies, Functional Ceramic Composites and Dispersed Superhard Materials V. Bakul Institute for Superhard materials National Science Academy Ukraine, Ukraine
3Department of Fire Prevention in Settlements, National University of Civil Defence of Ukraine, Ukraine
Correspondence: Sergii Guzii, Department of Nuclear Physics Technologies, State Institution, The Institute of Environmental Geochemistry of National Academy of Sciences of Ukraine
Received: February 12, 2024 | Published: March 28, 2024
Citation: Sergii G, Oksana A, Lyudmila O, et al. Magnetite-ferrocyanide-copper sorbents for recovery of cesium ions from low-activity liquid radioactive waters. Material Sci & Eng. 2024;8(1):15‒18. DOI: 10.15406/mseij.2024.08.00230
The article is devoted to the development of nanoscale sorbents based on copper ferrocyanide and magnetites for the removal of cesium, strontium, and heavy metal ions in their simultaneous presence in a multicomponent two-phase low-level radioactive solution containing complexing agents and surfactants. It they noted that the highest values of cesium ion removal from low-level radioactive water - 99.96% and 99.62% - are obtained with sorbents based on 100% copper ferrocyanide and a mixture of copper ferrocyanide and industrial magnetite in a ratio of 2 to 1. The efficiency of the sorbents in terms of the distribution coefficient is 2394 and 1589.7 ml/g, which ensures the purification of radioactive water from trace concentrations of cesium ions to values of 2394 and 265.11. These sorbents provide water purification to values less than 2 Bq/cm3, which is typical for 1-4 classes of water quality in terms of radiation safety of groundwater and surface water as sources of centralised drinking water supply. It is noted that sorption of cesium ions on copper ferrocyanide and magnetite is carried out in the presence of water molecules and hydroxo groups. It is shown that artificial magnetite in its pure form exceeds the sorption capacity of industrial magnetite. This is due to the difference in the phase composition and particle size of the crystals. The results obtained will be use as components of the technological process in the Plasma-Sorb technology developed at the State Institution "The Institute of Environmental Geochemistry" of the National Academy of Sciences of Ukraine for the treatment of low- and intermediate-level radioactive water from Ukrainian nuclear power plants.
Keywords: complex sorbents, copper ferrocyanide, low-activity liquid radioactive water, nanomagnetite, radionuclides, sorption
One of the main environmental problems in the 30-kilometre exclusion zone of the Chornobyl NPP is the processes of radionuclide transfer (migration) through groundwater and groundwater. The active source of radioactive isotopes in the Exclusion Zone groundwater (cesium, strontium, uranium, plutonium and americium) is the temporary radioactive waste containment sites (TRS), which are burial sites for parts of buildings, metal structures, radioactive soil, forest, etc.1 To date many methods of cesium ion extraction they known, but practice shows that the most effective is the combination of several methods and the achievement of selective extraction of 137Cs. The most common methods are adsorption, ion exchange, chemical precipitation, chemical reduction, membrane technologies, coagulation, extraction, and ion flotation.2–5 Adsorption methods have become the most widely used in liquid radioactive waste decontamination technologies due to a wide range of adsorbents, process efficiency, simplicity of technology and a wide range of applications.6,7 Among natural minerals, clinoptilolite, vermiculite, and montmorillonite we most often used for radionuclide sorption, but their main disadvantage is a relatively low sorption capacity.1,4
The most effective sorbents for cesium extraction are ferrocyanide sorbents8–11 and sorbents based on magnetite nanoparticles12–14 and complex magnetite-silicate-zeolite sorbents15,16 which are quite promising for the selective removal of cesium (up to 80 %) and strontium (up to 90 %) radionuclides from contaminated waters by magnetic separation. A particularly promising technology for radioactive water purification is the application of Plasma Sorb technology, which uses multi-stage purification not only by physical methods, but also by complex sorbents, including ferrocyanides, iron nanoparticles and other components.17 The application of this technology allows obtaining technical water that meets the requirements of the DSTU 4808:2007 standard in terms of its indicators (Sources of the centralized drinking water supplying. The hygienic, ecological requirements to water quality and the rules of selection, Ukraine). Therefore, the development and implementation of ferrocyanide-magnetite sorbents is an urgent issue to reduce the amount of hazardous liquid radioactive waste and potential environmental risks in their management.
Low-level radioactive water they prepared by the acid method from soil collected in the suburbs of Fokushima near the NPP.18 A 1 g soil sample we dissolved in 10 ml of 40% HF overnight, 700 ml of tap water we added while stirring, and the pH we adjusted to a value not exceeding 8.5 with NaOH solution. The concentration of sodium ions was 0.09 mol/l. This concentration of sodium ions does not affect the sorption of 137Cs ions.
According to X-ray fluorescence analysis, Fukushima soil contains the following chemical elements, %: O – 0.077; Al – 0.129; Si – 0.08; P – 0.017; S – 0.008; K – 0.051; Ca – 0.034; Ti – 0.011; V – ppm 446±46; Cr – ppm 163±20;Mn – 0. 003; Fe – 0.029; Ni – ppm 168±7; Cu – ppm 201±5; Zn – 0.001; Ga – ppm 93±4; As – ppm 98±6; Rb – ppm 369±6; Sr – 0.001; Y – ppm 108±7; Zr – 0.001; Nb – ppm 37±5; Ba – ppm 323±68; Pb – 0.002. In terms of oxides, wt. %: Al2O3 – 10.436; SiO2 – 46.861; ZrO2 – 0.11; Nb2O5 – ppm 52; P2O5 – 1.492; SO3 – 2.514; K2O – 5.464; CaO – 8.095; Cr2O3 – ppm 238; Ni2O3 -ppm 236; MnO2 – 0.546; Fe2O3 – 21.318; CuO – ppm 252; ZnO – 0.22; TiO2 – 2.508; V2O5 – 0.08; Ga2O3 – ppm 125; As – ppm 130; Rb2О – ppm 404; SrO – 0.069; Y2O3 – ppm 138; BaO – ppm 361; PbO – 0.094.
Copper ferrocyanide, nanopowders of artificial magnetite (Figure 1) and industrial magnetite concentrate (Poltava GOK, Ukraine) (Figure 2), and their mixtures were used as sorbents. Copper ferrocyanide we prepared by solid phase reaction by grinding ferric ferrocyanide with copper sulphate in a porcelain mortar for 30 min:
2CuSO4×5H2O + K4[Fe(CN)6]×3H2O = Cu2[Fe(CN)6]×7H2O + 2K2SO4 + 6H2O
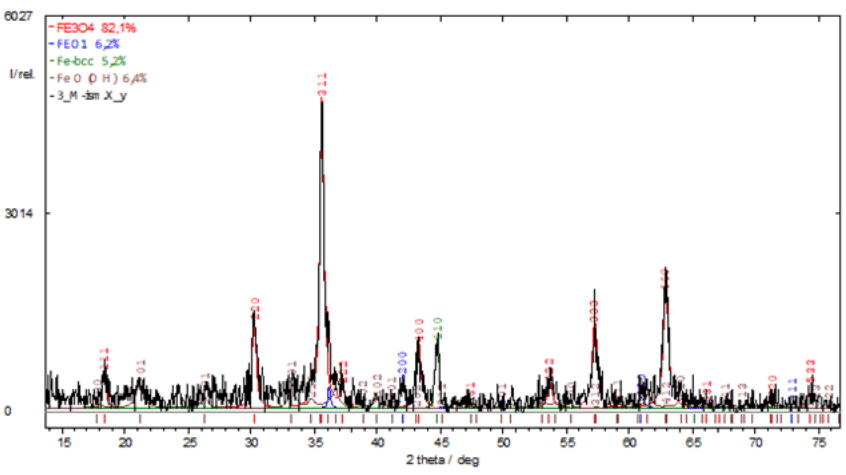
Figure 1 X-ray diffraction pattern of nanopowder containing polyvalent iron oxides obtained by electrical discharge dispersion (EDD).
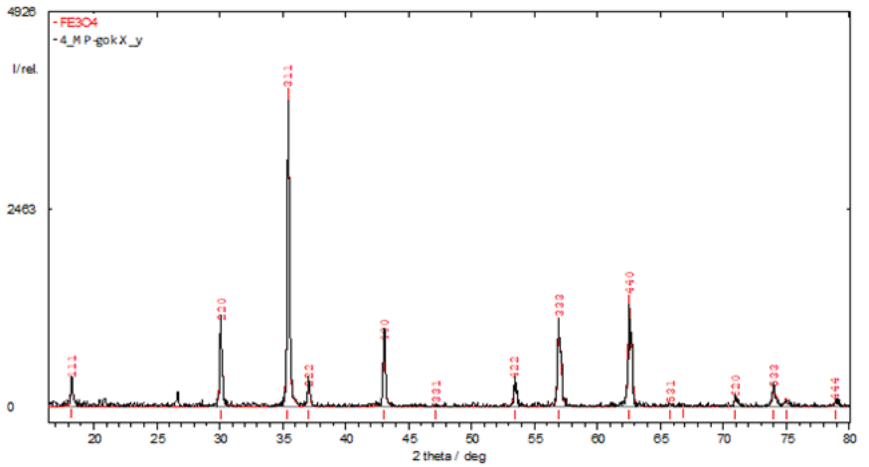
Figure 2 X-ray diffraction pattern of industrially produced magnetite nanopowder (Poltava GOK, Ukraine).
Artificial magnetite19 was produced by electro-erosive dispersion (EED) in plasma of sawdust from St3 or St40 steel (ISM NASU, Ukraine), American analogues of M1017 and 1040 steels. The phase composition of the nanopowder is as follows: Fe3O4 - 82.1%; FeO - 6.2%; Fe-bcc - 5.2%: FeO(OH) - 6.4% (Figure 1).
The degree of purification of weakly active radioactive water from cesium ions we calculated by the formula:20
Where Ai, Af - initial and final activity of radioactive water, Bq
The distribution coefficient we calculated according to the formula:
Where V - volume of liquid phase, ml; m - sorbent weight, g
The purification factor we calculated according to the formula:
For purification of radioactive water, sorbents were administered at a ratio of 1:10, i.e. 4 (6) g per 400 ml of water (4 g - 100%, 6 g - 100% copper ferrocyanide +50% magnetite). The activity of the compound samples (ARR) we measured using a FoodLight radiometer (developed by State Institute "Institute of Environmental Geochemistry" of National Academy of Sciences of Ukraine) and Gamma radiation spectrometer "ATOLL-3M" (Company Experience NPMSP, SP, Kyiv, Ukraine).
The results on purification of weakly active radioactive water from caesium ions by sorbents based on copper ferrocyanide and magnetite, as well as by complex sorbents based on them, we shown in Figure 3 & 4, Table 1. As can be seen from Figure 1 & 2, g-bursts on activity of caesium ions in water are close to the natural background, which confirms the correct choice of sorbents. The highest degree of purification of radioactive water from caesium ions - 99.96% and 99.62% it’s provided by sorbents based on copper ferrocyanide (100%) and a mixture of copper ferrocyanide (100%) with 50% of natural magnetite (Table 1). In terms of distribution coefficient, the efficiency of sorbents is placed in the following range: 100% СFС>100% AM>100% IM>100% CFC+50% IM>100% CFC+50% AM, which corresponds to 2394>2373>2331.5>1590.7>1589.7 ml/g (Table 1).
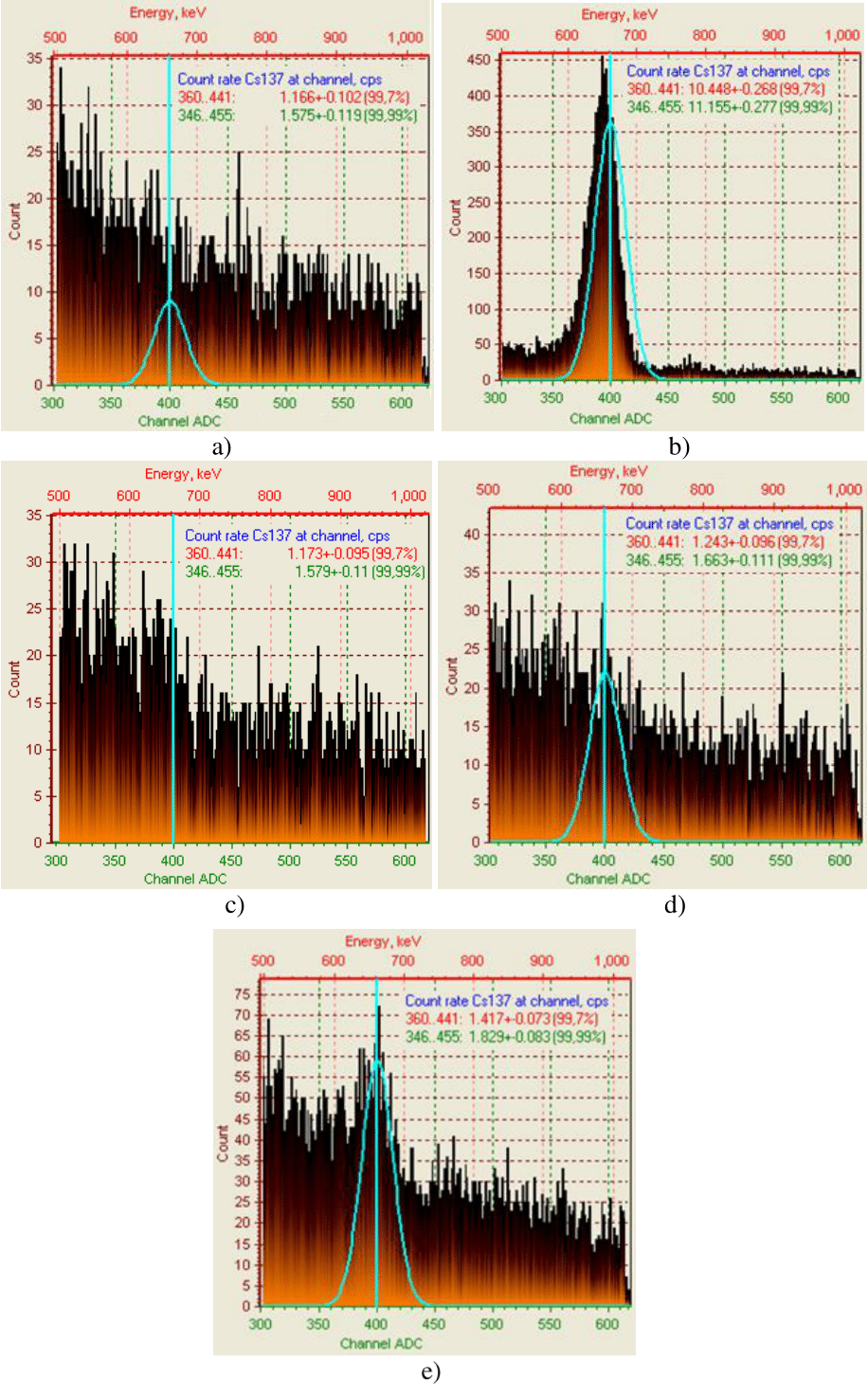
Figure 3 Intensity of γ-spectrum for 137Cs+ of weakly active radioactive water after treatment with sorbents: a - background; b - initial radioactive water; c - 100% CFC; d - 100% AM; e - 100% IM.
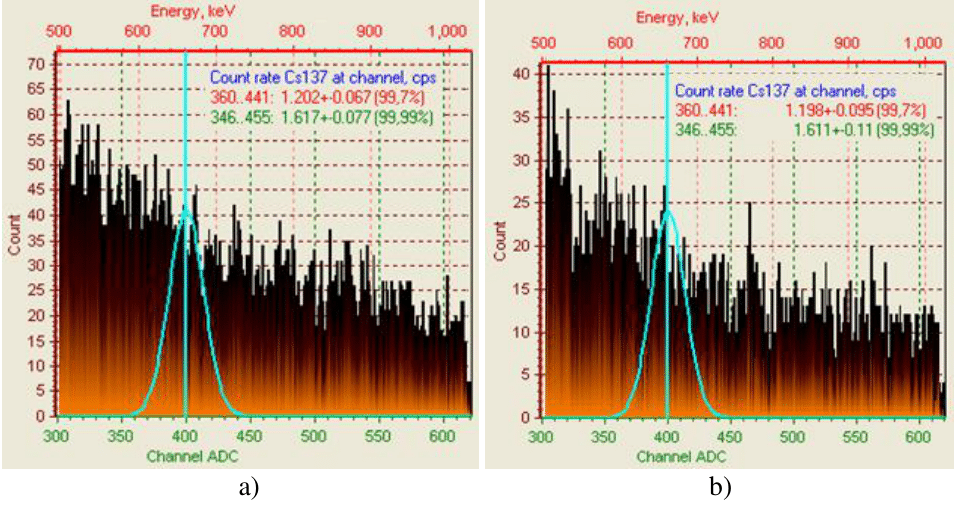
Figure 4 Intensity of γ-spectrum for 137Cs+ of weakly active radioactive water after treatment with sorbents: a - 100% CFC+50% AМ; b - 100% CFC+50% IM.
|
Sorbent |
A, Bq/cm3 |
S, % |
Kd, ml/g |
Kp |
|
RW |
23.95 |
- |
- |
- |
|
100% СFС |
0.01 |
99.96 |
2394 |
2394 |
|
100% AM |
0.22 |
98.96 |
2373 |
107.86 |
|
100% IM |
0.635 |
97.35 |
2331.5 |
36.72 |
|
100% CFC+50% AM |
0.105 |
99.56 |
1589.7 |
227.1 |
|
100% CFC+50% IM |
0.09 |
99.62 |
1590.7 |
265.11 |
Table 1 Cesium ion exchange characteristics of ferrocyanide-magnetite sorbents
Note: RW, initial radioactive water; CFC, Cu ferrocyanide; AM, artificial magnetite; IM, industrial magnetite.
According to the coefficient of purification of weakly active radioactive water from trace concentrations of caesium ions sorbents can be placed in a number as follows: 100% СFС>100% CFC+50% AM > CFC 100% +50% IM> 100%AM> 100% IM, which corresponds to the values 2394>265.11>227.1>107.86>36.72 (Table 1). According to the data of measuring the activity on the Atoll-3M device of a water sample after its purification with a sorbent of the 100%CFC+50%M clade, complete sorption of cesium ions is recorded (Table 2 and Figure 5).
|
Channel |
Energy, keV |
HWHH*, кеV |
Area |
Unrecognisable area |
Nuclides |
Activity, Bq |
|
219,194 |
668,405 |
67,450 |
-217 |
229 |
Cs-137 |
-3 |
|
469,622 |
1435,185 |
77,181 |
4121 |
185 |
K-40 |
385 |
Table 2 Water measurement on the Atoll-3M device
*Peak half-width and half-height.
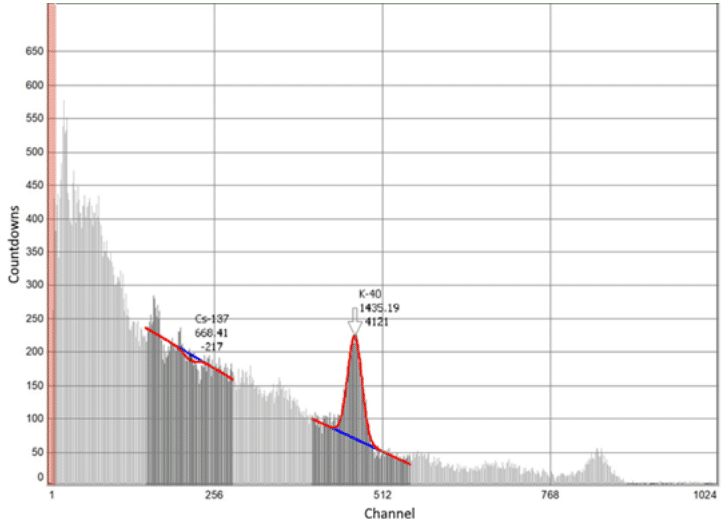
Figure 5 Intensity of γ-spectrum for 137Cs+ weakly radioactive water after its treatment with sorbent 100% CFC+50% IM.
The classification of groundwater and surface water quality as sources of centralized drinking water supply according to the environmental indicator, namely the radiation safety criterion, they given.21 The range of values of water quality indicators (criteria) we divided into four classes: Class 1 - excellent, desirable water quality; Class 2 - good, acceptable water quality; Class 3 - satisfactory, acceptable water quality; Class 4 - mediocre, limitedly suitable, undesirable water quality. In the Table 3 summarizes the data for compliance with the requirements of the standard21 activity of treated water in terms of caesium ion content. As can be seen from the data of Table 3, the developed compositions of sorbents provide water purification from cesium ions of all 4 classes. On sorption of strontium ions, the question is open. The developed compositions of sorbents are capable of sorption of strontium ions. However, in sediments, after sorption of caesium ions, no b-radiation we observed. Therefore, there is an assumption that strontium ions remained in the composition of water or evaporated during drying of the sediment.
|
Type of Sorbent |
Water quality classes by 137Cs, Bq/cm3 [21] |
|||
|
1 |
2 |
3 |
4 |
|
|
< 2 |
< 2 |
< 2 |
< 100 |
|
|
100% СFС |
0.01 |
0.01 |
0.01 |
0.01 |
|
100% AM |
0.22 |
0.22 |
0.22 |
0.22 |
|
100% IM |
0.635 |
0.635 |
0.635 |
0.635 |
|
100% CFC+50% AM |
0.105 |
0.105 |
0.105 |
0.105 |
|
100% CFC+50% IM |
0.09 |
0.09 |
0.09 |
0.09 |
Table 3 Radiation safety indicators
According to the results of the performed work, it we established that the highest degree of purification of weakly active radioactive water from caesium ions – 99.96% and 99.62% is provided by sorbents based on copper ferrocyanide (100%) and a mixture of copper ferrocyanide (100%) with 50% of industrial magnetite. By the value of distribution coefficient (Kd) the efficiency of sorbents is in the following range: 100% СFС>100% AM>100% IM>100% CFC+50% IM>100% CFC+50% AM, which corresponds to the values 2394>2373>2331.5>1590.7>1589.7 ml/g. By the coefficient of purification of weakly active radioactive water from trace concentrations of caesium ions, the sorbents are located in the following row: 100% СFС>100% CFC+50% AM > CFC 100% +50% IM> 100%AM> 100% IM, which corresponds to the values 2394>265.11>227.1>107.86>36.72. The developed compositions of sorbents provide water purification from caesium ions of 4 classes of water. Sorption of caesium ions on copper ferrocyanide and magnetite they carried out in the presence of water molecules and hydroxo groups. It is shown that artificial magnetite in pure form exceeds the sorption capacity of industrial magnetite. This is due to the difference in phase composition and particle size of crystals.
The work we carried out within the framework of the contractual topic at the State Institution Institute of Environmental Geochemistry of the National Academy of Sciences of Ukraine No. 001/2023-d of 23.03.23 "Scientific support of research using PLASMA-SORB technology for the processing of liquid radioactive waste".
Manuscript has no associated data.
The authors confirm that they did not use artificial intelligence technologies when creating the current work.
The authors express their gratitude to the staff of the State Institution "Institute of Environmental Geochemistry" of the National Academy of Sciences of Ukraine Sergii Shpilka and Senior Researcher PhD Volodymyr Burtnyak for their assistance in measuring the activity of low-radioactive water samples with developed compositions of complex inorganic sorbents.
The authors declare that they have no conflict of interest in relation to this research, whether financial, personal, authorship or otherwise, that could affect the research and its results presented in this paper.

©2024 Sergii, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.