eISSN: 2574-9927


Research Article Volume 4 Issue 1
1Department of Powder Metallurgy, Welding, and Materials Technology, Belorussian National Technical University, Belarus
2Department of Technical Physics, Belorussian National Technical University, Belarus
3National Science Center ‘Kharkov Institute of Physics and Technology’, Ukraine
Correspondence: Goltsova MV, Department of Powder Metallurgy, Welding, and Materials Technology, Belorussian National Technical University, Minsk, Belarus
Received: February 04, 2020 | Published: February 11, 2020
Citation: Goltsova MV, Zhirov GI, Tolmacheva GN. Nanoindentation of palladium hydride. Material Sci & Engl. 2020;4(1):20-24. DOI: 10.15406/mseij.2020.04.00121
For decades hydrides were considered as brittle matters. Different experiments in model palladium-hydrogen system fulfilled in conditions in which hydride phase transformations took place confirmed this statement. We elaborated technique of palladium hydrogenation by the way ‘out’ of binary state cupola, so that saturated with hydrogen palladium does not undergone hydride phase transformation. Using the specially constructed hydrogen-vacuum device and well known methods of tensile tests and nanoindentation we have shown that palladium hydride can be produced as material whose plasticity is as high as plasticity of pure annealed palladium and nanohardness of which is even 30% softer than that of pure annealed palladium. This is a fundamental result, changing the ideas about the nature of palladium hydrides properties.
Keywords: hydrogen, palladium, hydrides properties, hydrogen-metal interaction, β-phase, nanohardness
HPN, hydrogen phase hardening; HVD, hydrogen-vacuum device; HC, hydrogen concentrational; β-PdHx, β-hydrides of palladium
The Pd-H system is a unique one. High hydrogen permeation in palladium allows to use palladium like a membrane material to extra pure hydrogen gas production, as catalyzer for hydrogen penetration promotion in different metals, etc. For aims of hydrogen energy development, in which different types of Me-H systems are applied, most important feature of Pd-H system is in the fact that it is a classic system for hydrogen-metal interaction modelling. Really, all the Me-H systems have rather complicated equilibrium diagrams, and just thermodynamically opened Pd-H has a simple one,1 which looks like binary state cupola in coordinates T,oC, P, MPa, n(H/Pd). Despite the fact that Pd-H is under investigations for almost 200 years, this system is still full of surprises for researchers.1 The simplicity of Pd-H diagram is as follows. Under conditions corresponding to the left of the binary state cupola region (Figure 1), there is a dilute solid solution of hydrogen in palladium which is named α-phase. To the right of the cupola there is a saturated solid solution of hydrogen in palladium, (denoted as β- phase, rarely α'-phase). If the figurative point of the sample crosses the two-phase region, a hydride transformation develops in the sample. Hydride transformation products cannot be detected by etching, so their morphology is studied by investigating the development of surface relief on a pre-polished metallographic cross-section in an optical microscope in oblique lighting.2 It was discovered3 that during both α®β and β®α hydride transformations, the previously polished metallographic section is irreversibly deformed. As a result of hydride phase transformations, metals and alloys strongly harden and all their physical properties change. This phenomenon was called "hydrogen phase hardening" (HPN).4 If, however, the palladium sample is hydrogenated up to β-hydride state by the way “out” of the two-phase cupola, i.e. by such a way that the figurative point of the sample does not intersect the two-phase region, then the hydride transformation does not develop and the metallographic cross-section remains generally unchanged.2 As there were no phase transformations2 proceeding by the way “out” of the cupola, the sample saves its preliminary annealed structure, and has β-phase through all its volume. So samples hydrogenated by the technique ‘out’ of the binary state cupola we name β-hydrides of palladium (β-PdHx). Mechanical properties are one of main features characterizing materials in general and metal-hydrogen alloys particularly. Classic techniques on mechanical properties study used by specialists were always tensile tests, hardness measurements and so on. After the pioneer work of Oliver and Farr on nanoindentation was published,5 through decades the nanoindentation technique became a tool for the measurement of mechanical properties at small scales and even can have greater importance in science as a technique for experimental studies of materials physics fundamentals.6 Despite the fact that nowadays this method is widely used for a large variety of materials, we could find an only work on nanoindentation of palladium-hydrogen, by J.M. Wheeler and T.W.Clyne.7 In that work nanoindentation had been used to track the mechanical effects of hydrogen on palladium foils over a range of hydrogen concentrations. There were electrolytically fulfilled hydrogenation/dehydrogenation cycles in conditions through binary state cupola on the Pd-H diagram and was found that nanoindentation can measure the extent of hydrogen-induced phase transformations across the film thickness after hydrogen removal, with the α → β → α phase transformations yielding a ∼50% increase in local hardness. The aim of our work was to fulfill palladium hydrogenation from the gaseous phase by the way ‘out’ of the binary state cupola on the Pd-H diagram up to hydride of palladium β-PdHx state. Then we targeted identifying different mechanical properties of the produced by this way β-PdHx by classic tensile tests and by nanoindentation.
We used pure palladium samples (99,98%) of different forms due to different experimental tasks.
Mechanical measurements
Samples of 165mm length and of Æ0.5 mm were used. They were preliminary annealed in vacuum at 750°C for 0.5 hour. Average grain size after annealing was ~50cm. We used specially designed hydrogen-vacuum device HVD-3 which was modernized for hydrogen treatment of long samples. Structural scheme of HVD-3 is shown on Figure 2. The HVD-3 makes hydrogenation of samples up to 200mm of length possible at gaseous hydrogen pressures up to 4 MPa and temperatures up to 1100оС. Samples hydrogenation was controlled by electrical resistance measurements of reference specimen of 45mm length. Tensile tests were fulfilled at room temperature on the tensile tests machine with maximum intension of 500N. There were taken three samples for one experimental point. Before carrying out mechanical tests, marks with a pitch of 5mm were applied to the working part of the samples. Length of samples working part was 100mm. The stretching of the samples was carried out at a constant speed of moving the mobile clamp of the tensile machine - 10mm/min, at the limit of the load scale equal to 10kg. After tests there were calculated mechanical characteristics of palladium and its hydride with standard methods: strength limit sв, N/mm2; yield strength s0.2, N/mm2; relative elongation d%. The instrumental relative measurement error was 1–2%. Mechanical properties tests were carried out at room temperature in air. Additional measurements of the electrical resistivity were made before and after mechanical tests for the β-PdHx samples. These studies confirmed that during the tests hydrogen content in the samples did not change. Palladium β-hydride elastic modulus and nanohardness were tested by method of nanoindentation.
Nanoindentation
For this part of experiments bulk palladium samples with the same purity of 99.98% and an area of 10x10mm were cut from a palladium plate of 2.7mm thick. One of the samples was left in the initial state after rolling, others were preliminary annealed at 700°C for 1hour to remove the hardening and residual stresses. Further metallographic sections were prepared on all the samples (their surface roughness did not exceed 100nm). The two of the samples (the initial and annealed one) were immediately tested on the Nano Indenter G 200.Nano Indenter G 200 is well known device to determine the near-surface properties of material structures in micro- and nano-ranges. The tests were carried out with a Berkovich pyramid with a radius at the pyramid top of 230nm at a constant strain rate of 0.05s-1 and a maximum insertion depth of 2000nm. The resolution of G200 is less than 0.01nm; resolution of loading is 1÷50nN. To calculate the average value, 10 tests were performed on each sample. Other annealed samples with prepared surface metallographic sections on their top were saturated with hydrogen under conditions ‘out’ two-phase (α+β) –region as following to scheme shown in Figure 1. For this purpose, the hydrogen-vacuum device HVD-3 was also used.
“Out” of the two-phase cupola hydrogenation technique
The scheme for samples hydrogen treatment is shown in Figure 1. After vacuumation (point 1 in Figure 1), the samples in the working chamber were heated to 350°C (point 2 in Figure 1). This temperature is 58°C higher than Pd-H critical temperature Tc (292oC). Then hydrogen was given into the working chamber up to a pressure of 2,3MPa. The average hydrogen feed rate was 0.15-2MPa/min. The figurative point of the system accordingly moved from point 2 to point 3 (Figure 1).Under conditions (350°C, 2.3MPa), wire samples for mechanical tests were held for 10 minutes (in case of bulk samples hydrogen treatment for nano-measurements, the exposure duration was 20 minutes). Then, at a constant hydrogen pressure of 2.3MPa, samples were cooled to room temperature. The cooling rate was 2-4°C/min (figurative point motion along the isobar I from 3 to 4 in Figure 1). Thus, along the isobar of 2.3MPa, the figurative point of the system moves behind the two-phase cupola. The slow cooling rate must ensure the absence of internal thermal and hydrogen concentration stresses generation. After cooling to room temperature (4 in Figure 1), hydrogen was evacuated from the working chamber at a rate of 0.05-0.1MPa / min and then samples were taken out from the device. β-PdHx produced by such a technique had, according to the phase diagram1 and our estimates, a composition of PdH0.69 (that is, for every 100 atoms of palladium there are 69 hydrogen atoms in the alloy). Wire samples were immediately undergone tensile tests. What about bulk samples for nanoindentation, hydrogenated samples were placed in the working chamber of the Nano Indentor G200 immediately after removal from hydrogen, but due to the Nano Indenter G200 measuring technology undergone nanoindentation in 12 hours.
The palladium in the cold-worked state (95% of deformation) had the following mechanical characteristics: sв=297 Н/mm2; s0,2=224 Н/mm2; d=1.1 %. Cold-worked palladium had nanohardness of 1.695GPа and elastic modulus of 133 GPa. After annealing at 750°C (0.5 hour) the mechanical properties of palladium turned out to be equal: sв=188 Н/mm2; s0,2=38 Н/mm2; d=33 %. The nanohardness and elastic modulus of annealed palladium were 1.213 and 128GPa, respectively. These results are in good agreement with the literature data8,9 and indicate that the palladium used in the work due to its high purity has low strength properties and a very high plasticity in the annealed state. After hydrogen treatment PdH0.69 samples undergone tensile tests immediately. The control measurements of the electrical resistivity before and after the tests confirmed that during the tests the hydrogen content in the alloys did not change. It was found that β-PdHx alloy obtained by the above-described technology with hydrogenation “out” of the two-phase cupola on the Pd-H diagram had the following mechanical properties sв=200 Н/mm2; s0,=31 Н/mm2; d=34 %. The surprising result here is that palladium hydride produced by the way “out” of the binary cupola is a highly plastic and low-strength material. Its mechanical properties are generally close to those of pure annealed palladium. In this case, palladium hydride (in comparison with pure palladium) has a strength limit of 6% higher than the ultimate strength of pure palladium, the conditional yield point is lower by ~ 22%, and the elongation is practically equal to the level of annealed palladium. Let us discuss this non-trivial result (usually hydrides are considered as highly fragile materials10 in the light of historical development of ideas about the nature of hydrides.
The term "hydride" was introduced in chemistry11 for chemical compounds of hydrogen with metals, like carbide, boride, nitride - for compounds of metals with carbon, boron and nitrogen. Chemical compounds usually have an accurate stoichiometric composition. Since they are characterized by a predominance of covalent (or ionic) bonds, such chemical compounds are usually highly fragile materials. Hydrides of transition metals and, in particular, palladium hydride, do not fit into these concepts. First of all, palladium hydride has a wide range of hydrogen solubility. Palladium hydride by its physical nature is not a chemical compound of a metal with hydrogen. It is a concentrated solid interstitial solution of hydrogen in palladium of variable composition, which has all the features of a metal alloy, i.e. a characteristic metallic sheen, high (metallic) electrical conductivity which decreases with temperature, and high metal plasticity. It is clear that palladium hydride, being a metallic material, can be subjected to metal-specific treatments, like hydrogen-hard work hardening, external plastic deformation, subsequent recrystallization, etc. Anyway, comparing mechanical properties of palladium β-hydride with properties of annealed pure palladium, we were surprised by extra high level of this material plasticity. So, the question which appeared was “did the dissolved hydrogen change fundamental metal characteristics such as for example elasticity modulus?”
Nanoindentation results fully answered this question. Figure 2 represents polished surface of pure annealed palladium (a) and surface of β-hydride of palladium after hydrogenation (b). It can be seen that when the bulk sample was hydrogenated, the grain boundaries appeared on the preliminary polished cross section, and slip bands appeared within the individual grains. This agrees with previously obtained data,2 in which grain boundaries appearance under hydrogenation on preliminary polished palladium surface was registered by in situ optical microscopy.2 The grains shift shows that a rate of bulk samples hydrogenation was not enough slow and caused the difference in hydrogen concentrations in outer and inner layers of the sample resulted in inner hydrogen concentrational (HC-) stresses. The latter were then relaxed by grains shift (for more details on this phenomenon, see work.12 The absence of a surface relief in the body of every grain indicates that the figurative point of the sample had bypassed “out” the two-phase region (Figure 3). The typical curves of load-displacement for β-PdHx are shown in Figure 4. We have found that palladium b-hydride elasticity modulus was less than the elasticity modulus of pure annealed palladium and was just 124.8GPa (compare with 128 of pure annealed palladium). The average value of b-PdH0.69 nanohardeness in thin layers (up to 2000 nm) was just 0.842GPa. And this value is much less (on 30% smaller) than the nanohardness of pure annealed palladium (1.213GPa)!
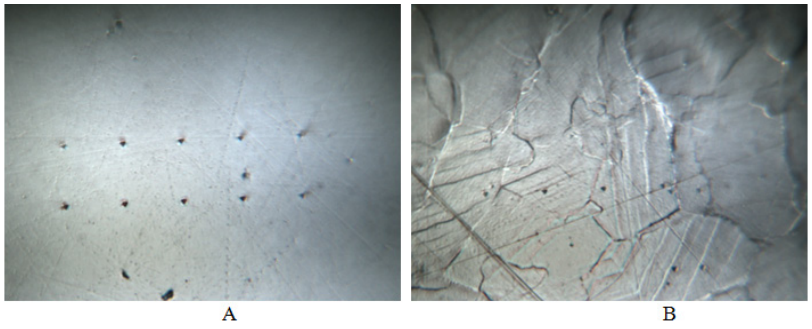
Figure 3 Cross section surfaces with Berkovich pyramid prints of pure palladium (A) and of -hydride of palladium produced by hydrogenating “out” of the two-phase cupola on the Pd-H diagram (B), 1000.
Remember that all the samples in our experiments were hydrogenated without proceeding hydride phase a®b transformations. Experimental data published earlier by others researchers give a probability to compare our results with those which were obtained with palladium hydrogenation through phase transformation fulfilling. So, Wheeler JM & Clyne TW7 showed that palladium experiencing α→ β→ α phase transformations showed a ∼50% increase hardness. The situation for partially hydrided samples having both α- and β- phases regions in structure is even more complicated – regions where α-phase was saturated with hydrogen showed a ∼75% increase in hardness.7 So, literature data for samples which undergone phase transformation is expectedly opposite. To make sure that the hydrogen content in the sample did not change during the time in which samples were tested in the Nanoindentor, we performed13 X-ray studies. b-PdH0.69 samples with dimensions (10´5´0.27)mm, obtained by the described above hydrogenation “out” of the binary state cupola technique were subjected to X-ray analysis immediately after saturation, then after 24 and 48 hours of exposure in air. There was used DRON-3 diffractometer, in filtered copper radiation (Cu ka=1.54181 Å, Cu ka1=1.54056 Å). The diffractometer was equipped with a program for digitizing X-ray spectra. Diffractograms were recorded under the same modes. The analysis (Figure 5) showed that the composition of the samples did not change within 24 hours after they were taken out from hydrogen. The first significant changes began after 48 hours’ exposure (Figure 5C). Therefore, indeed, there is a tendency to decrease the elasticity modulus of palladium hydrogenated “out” the binary state cupola.
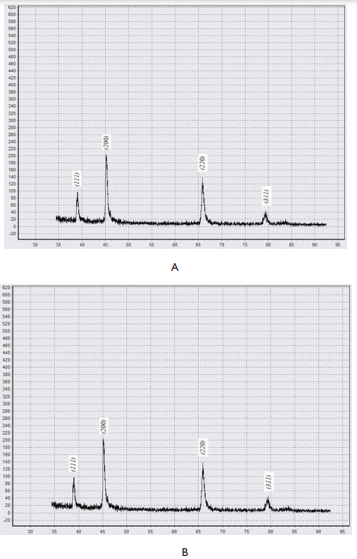
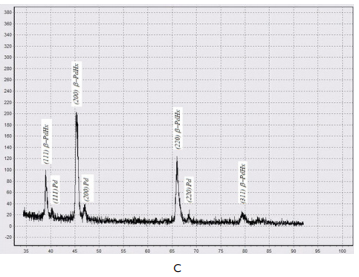
Figure 5 X-ray diffraction analysis of β-PdHx samples.13
A - Sample immediately after hydrogenating by way “out” the binary state cupola at the Pd-H state diagram; B - after 24 hours in the air exposure; C - after 48 hours in the air exposure.
Using the fact that grain boundaries were clearly visible on the surface of the cross section of β-PdHx, in the present work we had measured nanohardness and elastic modulus directly within the grain boundaries. The depth of indentation was up to 300nm. Figure 6 represents one of surface fragments with pyramid imprints (a) and relative load-displacement dependences (b). It was found, that grain borders average elasticity modulus is equal to 109GPa, that is much less than one measured in grain surface centers (124GPa). Meanwhile nanohardness of grain borders is equal to 0.905GPa, that is even a little bit more than average value of b-PdHx nanohardeness (0.842GPa). This experimental fact indirectly confirms our conclusion that hydrogen dissolved in palladium reduces the modulus of elasticity. As grains borders are potentially preferable places to hydrogen segregation, consequently their elasticity modulus is most reduced and it contributes to the overall increase in the softness of the b-PdHx.
We have produced the very plastic palladium hydride β-PdHx whose plasticity (relative elongation) is the same as one of pure annealed palladium and nanohardness of which is 30% less than that of pure palladium. This fact is surprising as far as hydrides were always considered as brittle matters. The nature of palladium hydrides is different from the other hydrides nature. β-PdHx properties depend on the way of production, namely, did palladium undergone phase transformation during hydrogenation or not. It was firstly found in the present work that hydrogen makes grain borders weaker reducing its elasticity modulus. Small value of grain borders elasticity modulus contributes to overall b-PdHx properties.
M.V.G. elaborated experimental plan and hydrogenation technique. G.I.Z. fulfilled tensile tests. G.N.T. carried out nanoindentation. M.V.G. had analyzed results and wrote manuscript.
Authors thanks assistant professor E.N. Lyubimenko for participation in experiments.
The authors declare that they have no competing interests.

©2020 Goltsova, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.