eISSN: 2574-9927


Research Article Volume 5 Issue 1
1Daily Sourcing & Research SRL Bucharest, Romania
2Department of Applied Chemistry and Materials Science Bucharest, University POLITEHNICA of Bucharest, Romania
3Cosfel Actual SRL Bucharest, Romania
Correspondence: Sorin Mircea Axinte, Department of Applied Chemistry and Materials Science Bucharest, University POLITEHNICA of Bucharest, Romania, Tel +40726979887
Received: December 31, 2020 | Published: February 11, 2021
Citation: Paunescu L, Dragoescu MF, Axinte SM, et al. Nonconventional manufacture technique of cellular glass from recycled aluminosilicate glass-based waste. Material Sci & Eng. 2021;5(1):11-16. DOI: 10.15406/mseij.2021.05.00149
The paper aimed to experimentally produce by sintering/foaming at over 950ºC a cellular glass with excellent physical, mechanical and microstructural characteristics using glass wastes (soda-lime glass and halogen lamps bulb glass), coal ash and silicon carbide as a foaming agent. The paper originality consists in the use of microwave heating, which allows the achievement of low specific energy consumptions (below 1kWh/kg). The main characteristics of the best experimental variant were: apparent density of 0.29g/cm3, thermal conductivity of 0.072W/m·K and compressive strength of 2.4MPa being suitable for the use of the product as a thermal insulating material in construction.
Keywords: cellular glass, microwave heating, soda-lime glass, aluminosilicate glass, energy consumption.
The glass is a non-crystalline, often transparent solid, whose important physical and mechanical characteristics make it suitable for various applications such as packaging, housewares, building windows, vehicle components, electrical equipment, fibers in composite materials, etc. Glass waste, existing in very large quantities in the world and with high annual growth rates, determines the need for its recycling, especially as there are limitations to the reuse of glass in the industrial process of manufacturing new glass. A well-known recycling solution is foaming the glass waste to obtain porous, light, rigid materials, with a good mechanical strength, chemical stability, fireproof, low water permeability, non-toxic, resistant to rodents, insects, bacteria and acids, etc.1 All these excellent features of the cellular glass recommend it as a very good construction material (thermal and sound insulators, lightweight structural components, filters, absorbers, gas sensors, heat exchangers, floors and walls tiles, architectural panels, industrial roofs arrangements, lightweight aggregates, road and railway construction materials, abutments of bridges, airport runways, foundations, drainages, sport grounds, etc.2 The current paper was focused on recycling waste from glass bulbs of halogen lamps, high-temperature thermometers, thermally and electrically highly loadable film resistors. The market for halogen lamps has been developed in the last decade of the last century in the USA. Other manufacturers that appeared later are: China, South Korea, Taiwan, Japan, the Phillipines, Mexico, Germany and Hungary. Incandescent lamps remain dominant in the United States market, based on their substantial use in the large residential and transportation equipment markets.3
According to,4 the materials that compose the halogen lamp bulb are: soda-lime glass, borosilicate glass, alkaline earth aluminosilicate glass, fused silica and sodium resistant glass. An average chemical composition of the lamp bulb glass was approximately calculated based on the composition of each type of glass mentioned above:5 67.7% SiO2; 6.5% Al2O3; 4.0% MgO; 13.2% CaO; 1.5% B2O3; 3.5% Na2O; 0.4% K2O; 0.2% Fe2O3; 0.2% TiO2; 2.8% other oxides. The paper5 comparatively presents four manufacturing techniques of cellular glass using different types of glass waste and foaming agent. In the first experiments set, commercial soda-lime glass waste (99wt.%) and silicon carbide (1wt.%) were mixed with supplementary ratios of aluminosilicate glass waste (between 1-10 wt.%). The pressed powder mixture in a small stainless steel mold was sintered at 950ºC for 30 min. With the increase of the weight proportion of aluminosilicate glass waste in the range of 1-10%, the apparent density of the glass foam increased linearly from 0.20 to 0.31g/cm3 and the compressive strength increased from 0.5 to a maximum of 2.6MPa corresponding to the proportion of aluminosilicate glass waste of 5%, then decreased to 2.2MPa in the range of 5-10% aluminosilicate glass waste. Relatively high mechanical strength of the foamed material was attributed to the crystallization of wollastonite and diopside in struts. The other sets of experiment successively used soda-lime glass waste and coal fly ash (80/20 weight ratio) and cathode ray tube (CRT) glass waste, respectively. Commercial silicon carbide and silicon carbide-based waste (obtained from polishing the paper) and calcium carbonate, respectively, were successively used as foaming agents, but the glass foam samples characteristics were less good compared to those obtained when using aluminosilicate glass waste. Worldwide, the technique adopted for the production of cellular glass from glass waste is based on the conventional heating of the raw material in classical conveyor belt ovens. The energy sources are either electrical resistances or the burning process of fossil fuels. The industrial manufacture of cellular glass is commonly made from post-consumer container glass waste and less frequently, flat glass waste as raw material and black carbon, calcium carbonate, silicon carbide or glycerol as foaming agent, depending on the required type of cellular glass. The main companies in the world that produce cellular glass are: Misapor Switzerland, Pittsburgh Corning, Geocell Schaumglas and Glapor.7 Also, numerous experimental tests performed worldwide aiming new recipes and manufacturing techniques have used conventional methods of heat treatment.8–13
Since 2016, the Romanian company Daily Sourcing & Research Bucharest performed several experimental manufacturing cellular glass from glass waste and various aluminosilicate waste using the microwave radiation as the only energy source.2,14,15 Although known since the mid-20th century as a fast, "clean" and economical heating method of solid and liquid materials, microwaves have only been applied in drying and low-temperature heating processes. According to,16 only in the 1990s it was experimentally found that several types of materials (organics, ceramics, metals, polymers, glasses, etc.) are suitable for an efficient microwave heating. Despite this finding, the situation of the industrial application of microwave heating in the world has not improved, this being in various experimental stages.
The basic principle of foaming the glass-based raw material is to incorporate a foaming agent into a finely ground powder mixture. The mixture is heated to a high temperature at which the glass softens and its viscosity becomes adequate to capture the gaseous products released by oxidation or decomposition of the foaming agent. The gas bubbles are blocked in the viscous mass of the glass and by cooling form a specific porous structure.1 The foaming agent adopted in these experiments was silicon carbide and the heating method was that of using microwave radiation. The release of gaseous products when using silicon carbide (carbon dioxide and monoxide) occurs through its oxidation reactions (1) and (2), which are mainly active at over 900 ºC.17
SiC + 2O2 = SiO2 + CO2 (1)
SiC + O2 = SiO + CO (2)
The optimal method of microwave heating of a pressed powder mixture containing glass, whose application had the best experimental results, is the mixed heating method (predominantly direct and partially indirect).2 The application of this method involves the use of a silicon carbide and silicon nitride (80/20 weight ratio) ceramic tube (outer diameter of 125mm, height of 100mm and a wall thickness of 2.5mm) placed between the radiation emission source and the material subjected to heating. The ceramic wall of the tube allows a limitation of the electromagnetic wave penetration so that the direct contact with the material does not destroy the structure of its core during the rapid heating and foaming, as happens in the case of the direct microwave heating of glass. Part of the microwave field emitted through the waveguide of the oven side wall is absorbed by the wall of the microwave susceptible ceramic tube, which heats up intensely and indirectly transfers heat to the material by thermal radiation. The upper opening of the tube is covered with a ceramic lid of the same material like it. The thermal protection with the ceramic fiber mattresses of the side wall of the tube and the ceramic lid as well as with the bed of the same mattresses type that is placed at the oven base are very important to avoid heat loss outside and to ensure the energy efficiency of heating process. The control of the heating process of the material was carried out with a radiation pyrometer mounted above the oven at about 400mm. To visualize the upper surface of the heated material, the upper sheet wall of the oven was provided with a 30mm-diameter hole and also similar holes were made in the lid and the ceramic fiber protection layer of the lid. Figure 1 presents an overall image of the 0.8kW-microwave oven used for heating process (a), the SiC and Si3N4 ceramic tube (b) and the ceramic fiber mattresses protection of the tube containing the material (c).

Figure 1 The experimental microwave equipment.
A- Overall image of the 0.8kW-microwave oven; B - SiC and Si3N4 ceramic tube; C - ceramic fiber mattresses protection of the tube containing the material.
Materials
The materials that make up the dry powder mixture for cellular glass manufacturing were: commercial soda-lime glass waste (post-consumer container glass), aluminosilicate glass waste (halogen bulb glass waste), coal fly ash and commercial silicon carbide. The soda-lime glass waste was constituted from post-consumer colorless, green and amber container glass broken, ground in a ball mill and sieved at a grain size below 80μm. The glass waste was composed from equal weight ratios of colorless, green and amber glass (Table 1). The halogen bulb glass waste containing soda-lime glass, borosilicate glass, alkaline earth aluminosilicate glass, fused silica and sodium resistant glass had an average chemical composition which includes: 67.7% SiO2; 6.5% Al2O3; 4.0% MgO; 13.2% CaO; 1.5% B2O3; 3.5% Na2O; 0.4% K2O; 0.2% Fe2O3; 0.2% TiO2 and 2.8% other oxides. The waste was broken, ground in a ball mill and sieved at a grain size below 80μm. The coal fly ash used in experiments is an industrial by-product provided by the Paroseni thermal power station having the following chemical composition: 46.5% SiO2; 23.7% Al2O3; 7.9% CaO; 3.2% MgO; 6.0% Na2O; 4.1% K2O and 8.6% Fe2O3, determined by X-ray fluorescence. The initial grain size of coal fly ash was below 250μm. After its grinding in the ball mill and sieving the final dimension was reduced below 63μm. A commercial silicon carbide with the grain size below 6.3μm without other processing was used in experiments.
|
Composition |
Container glass waste type |
||
|
Colourless |
Green |
Amber |
|
|
SiO2 |
71.7 |
71.8 |
71.1 |
|
Al2O3 |
1.9 |
1.9 |
2.0 |
|
CaO |
12.0 |
11.8 |
12.1 |
|
Fe2O3 |
- |
- |
0.2 |
|
MgO |
1.0 |
1.2 |
1.1 |
|
Na2O |
13.3 |
13.1 |
13.3 |
|
K2O |
- |
0.1 |
0.1 |
|
Cr2O3 |
0.05 |
0.09 |
- |
|
SO3 |
- |
- |
0.05 |
|
All other oxides |
0.05 |
0.01 |
0.05 |
Table 1 Chemical composition of the glass waste18
Four experimental variants containing 36.5-63.0% soda-lime glass waste, 35.0-50.0% halogen bulb glass waste, 10% coal fly ash, 2.0-2.5% silicon carbide and 10% water addition (to facilitate the powder mixture cold pressing) were adopted. The components weight ratios of each variant are shown in Table 2.Using constant amounts of dry and wet raw material of 560 g and 616 g, respectively, these have been heat treated to obtain sintered and foamed products. The sintering/foaming temperature values were not pre-established, but they were determined during the experiments. The radiation pyrometer indication regarding the temperature stabilization of the heated material and the beginning of its slight decrease was the signal that the foaming process developed in the sample mass reached its head and that the microwave heating must be stopped. The functional parameters of the sintering/foaming process are shown in Table 3.
|
Component |
Variant |
|||
|
1 |
2 |
3 |
4 |
|
|
Soda-lime glass waste |
53.0 |
47.5 |
42.0 |
36.5 |
|
Halogen bulb glass waste |
35.0 |
40.0 |
45.0 |
50.0 |
|
Coal fly ash |
10.0 |
10.0 |
10.0 |
10.0 |
|
Silicon carbide |
2.5 |
2.5 |
2.0 |
2.0 |
|
Water addition |
10.0 |
10.0 |
10.0 |
10.0 |
Table 2 Experimental variants of cellular glass
According to the data in Table 3, the temperature of the sintering and foaming process had values between 952-960 ºC, the temperature increasing being determined by the weight ratio increase of waste from glass bulbs of halogen lamps from 35.0 to 50.0%. Due to the application of the mixed microwave heating method, the average heating rate had relatively high values (between 21.4-24.5 ºC/min), but not at the level reached in the case of the direct microwave heating (generally over 30 ºC/min).14,15 The specific energy consumption of the experimental process had low values (0.88-1.01 kWh/kg) confirming the excellent energy efficiency of the application of the mixed microwave heating method, predominantly by direct heating. These values of energy consumption are almost similar to those of conventional industrial manufacturing processes. The physical, mechanical and microstructural characteristics of the cellular glass samples were determined by common methods. The thermal conductivity was determined by the guarded-comparative-longitudinal heat flow (ASTM E1225-04 standard) and the apparent density was measured by the gravimetric method.19 The water absorption was determined by the water immersion method (ASTM D570 standard) and the porosity was calculated by the method of comparing the true and apparent density.20 The compressive strength was measured using a Stable Micro Systems TA XT Plus Texture Analyzer. The samples microstructure was examined with a Smartphone Digital Microscope and to investigate the crystallographic structure of the cellular glass, X-ray diffraction (XRD) was used according to the standard EN 13925 – 2: 2003 using a X-ray diffractometer Bruker-AXS D8 Advance with CuKα radiation. The main physical, mechanical and microstructural characteristics of the cellular glass samples are presented in Table 4.
|
Parameter |
Variant 1 |
Variant 2 |
Variant 3 |
Variant 4 |
|
Dry/wet raw material amount (g) |
560/616 |
560/616 |
560/616 |
560/616 |
|
Sintering/ foaming temperature (ºC) |
952 |
955 |
958 |
960 |
|
Heating duration (min) |
38 |
39 |
41 |
44 |
|
Average rate (ºC/ min) - heating - cooling |
24.5 5.4 |
24.0 5.4 |
22.9 5.6 |
21.4 5.5 |
|
Index of volume growth |
1.90 |
1.70 |
1.60 |
1.40 |
|
Cellular glass amount (g) |
543 |
542 |
544 |
545 |
|
Specific energy consumption (kWh/kg) |
0.88 |
0.90 |
0.94 |
1.01 |
Table 3 Functional parameters of the sintering/foaming process
According to Table 4, the four experimental samples had low apparent density values. The lowest value (0.24 g/cm3) belonged to sample 1, with the lowest proportion of aluminosilicate glass waste (35%), the highest proportion of soda-lime glass waste (53%) and also the highest proportion of foaming agent (2.5%). By comparison, sample 4 had the highest apparent density (0.33 g/cm3) given that the ratio between aluminosilicate glass waste and soda-lime glass waste was highest (50.0/36.5). Therefore, it has been confirmed that aluminosilicate glass waste favors the increase of the apparent density of a mixture that also contains soda-lime glass waste. Implicitly, the value of the samples porosity decreased with the increase of the apparent density and the value of their thermal conductivity increased with the increase of the apparent density. According to the results of the water immersion test, all four cellular glass samples had an extremely low water permeability (below 0.6%). The compressive strength had quite high values, above the average level of cellular glasses made from commercial soda-lime glass waste. The highest value of compressive strength (2.8MPa) corresponding to sample (1) with the lowest apparent density (0.24g/cm3) should be discussed. Figure 2 & Figure 3 should be seen to obtain an explanation of this situation. It should be mentioned that the four porous products experimentally manufactured expanded during the thermal process in an irregular way and in the end they did not have a clearly delimited geometric shape. The samples shown in Figure 2 were extracted from larger volumes of manufactured products. The non-similarity between their geometric shapes is exclusively the fault of the authors' team.

Figure 2 Pictures of the cellular glass samples. A– sample 1, heated at 952ºC; B – sample 2, heated at 955ºC; B – sample 3, heated at 958ºC; d – sample 4, heated at 960ºC.
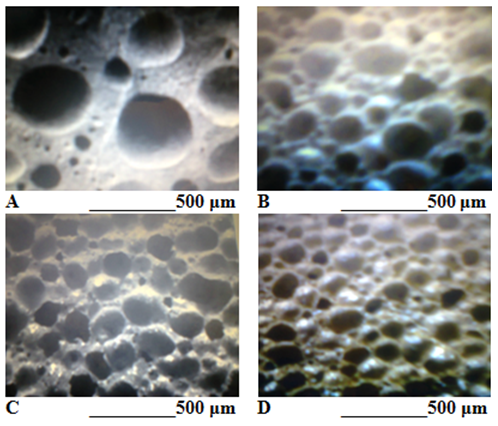
Figure 3 Microstructural images of the cellular glass samples. A – Sample 1; B – Sample 2; C – Sample 3; D – Sample 4.
Variant |
Apparent density g/cm3 |
Porosity % |
Thermal conductivity |
Compressive strength |
Water absorption |
Pore size mm |
1 |
0.24 |
87.1 |
0.058 |
2.8 |
0.4 |
0.13 – 0.41 |
2 |
0.27 |
85.7 |
0.065 |
2.5 |
0.6 |
0.16 – 0.29 |
3 |
0.29 |
84.8 |
0.072 |
2.4 |
0.4 |
0.15 – 0.25 |
4 |
0.33 |
83.8 |
0.075 |
2.3 |
0.3 |
0.10 – 0.22 |
Table 4 Physical, mechanical and morphological characteristics of the samples
It can be seen that the porosity level of sample 1 is the highest, instead the microstructure of the same sample indicates the existence of thick walls between cells, which contribute to the increase of the compressive strength of this cellular glass. The microstructural images of samples 2-4 in Figure 3 show a remarkable homogeneity of pore distribution. The sample with the lowest size pore (0.10-0.22 mm) is sample 4, but also the other samples (2 and 3) have low size pore (0.15-0.25mm and respectively, 0.16-0.29mm) being optimal in terms of feature insulating material. The analysis of the physical, mechanical and microstructural characteristics of the four cellular glass samples led to the selection of sample 3 as the optimal sample. The decisive criterion for selecting sample 3 as the optimal sample was the best uniformity of pore size (0.15-0.25mm) and the most homogeneous pore distribution (Figure 3C). Although it had the lowest density and thermal conductivity and the highest compressive strength, sample 1 had an inadequate microstructure with a wide pore size range (0.13-0.41mm). According to Table 2, sample 3 was made of 42% soda-lime glass waste, 45% halogen bulb glass waste and 10% coal fly ash as raw materials, 2% silicon carbide as a foaming agent and 10% water addition, by sintering and foaming at 958 ºC. The physical and mechanical characteristics of the porous product were: apparent density of 0.29 g/cm3, porosity of 84.8%, thermal conductivity of 0.072W/m·K and compressive strength of 2.4MPa. The pore size was between 0.15-0.25mm and the water absorption was of only 0.4%. All these characteristics of the material recommend it as a very good thermal insulator made of glass waste and an industrial by-product (coal fly ash). The technique of heat treatment by predominantly direct microwave heating led to a low level of the specific energy consumption (0.94kWh/kg). The investigation of the crystallographic structure of cellular glass sample 3 allowed to identify the presence of the crystalline phases: mainly, wollastonite and secondary, diopside and mullite (Figure 4).

Figure 4 The sample 3 XRD analysis (schematic presentation). 1 – Wollastonite; 2 – Diopside; 3 – Mullite.
Discussion
Unlike the conventional heating of solids in which the heat transfer from the energy emitting source to the material subjected to heating is performed by conductivity, convection and thermal radiation and the massive components of the oven (walls, vault, hearth, etc.) take over some of the heat for then transmits it to the material, the direct microwave heating takes place practically in reverse. The source emitting electromagnetic waves (microwaves) comes in direct contact with the material that must be, or contain at least one microwave susceptible component.21 The waves penetrate the material and their energy is converted into heat, initiating heating in the core of the cold material, where the maximum temperature of the heating process develops rapidly. Practically, under electromagnetic waves influence, the material itself becomes an energy sources.22 Experimentally, it has been found that the commercial glass used as a raw material, alone or mixed with other types of glass, is not suitable for the direct heating due to the high intensity of the conversion of microwave energy into heat that severely affects the inner structure of the glass.23 Therefore, a technique for tempering the direct contact of the microwave field with the material was applied in the Daily Sourcing & Research company2 by placing between the source and the material a screen made of a high microwave susceptible material (silicon carbide and silicon nitride in 80/20 weight ratio), which predominantly allows its penetration for direct heating of the material and partially absorbs in its wall part of the emitted microwave field. A low thickness of the screen wall (2.5mm) completely avoids destruction by direct heating of the structure of the glass-based powder mixture. In addition, the material also benefits from the thermal energy transmitted by radiation by the hot inner surface of the intensely heated ceramic screen following the absorption of the electromagnetic waves converted into heat. In the case of mixed microwave heating of the cold material, it is practically about the direct heating of both the material and the screen (ceramic tube or crucible), which in turn generates the indirect heating of the same material. The selective character of direct microwave heating is also kept in the case of predominantly direct mixed heating, in the sense that other components of the oven do not have to be heated to obtain the material heating as in the case of the direct heating.21 The main advantage of microwave heating of solids applied in the experiments described above is the low level of specific energy consumption of the process. According to,16 the use of microwave equipment on an industrial scale would allow to obtain a higher energy efficiency by up to 25% compared to that of the low power-microwave oven (0.8kW) used in experiments.
The paper aimed to experimentally manufacture a cellular glass with excellent physical, mechanical and microstructural characteristics obtained by sintering and foaming at over 950ºC in a 0.8kW-microwave oven of a wet pressed mixture containing glass wastes (a soda-lime glass waste and a halogen bulb glass waste), an industrial by-product (coal fly ash), a foaming agent (silicon carbide) and a water addition. Four experimental variants were tested, of which the variant that used 42% soda-lime glass waste, 45% halogen bulb glass waste, 10% coal fly ash, 2% silicon carbide and 10% water addition was adopted as the optimal variant. The sintering temperature had the value of 958 ºC, being reached in 41 min with the average heating rate of 22.9 ºC/min. The main characteristics of the optimal porous product were: apparent density of 0.29 g/cm3, porosity of 84.8%, thermal conductivity of 0.072W/m·K and compressive strength of 2.4MPa. The pore size was between 0.150.25mm and the water absorption was of only 0.4%. These characteristics are suitable for the use of the product as a thermal insulating material in construction. The originality of the paper consists in the adoption of the nonconventional technique of microwave heating, which allows the achievement of low specific energy consumptions (below 1kWh/kg) compared to the conventional techniques still preponderantly used in the world.
The research was fully sponsored by Daily Sourcing & Research SRL Bucharest, Romania.
None.
The authors declare that there is no conflict of interest.

©2021 Paunescu, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.