eISSN: 2379-6367


Research Article Volume 12 Issue 1
1Pharmaceutical Sciences Department, School of Pharmacy, Medical Science Campus-University of Puerto Rico, Puerto Rico
2Department of Pharmacology and Toxicology, School of Medicine, Medical Science Campus-University of Puerto Rico, Puerto Rico
Correspondence: José G Ortiz, Department of Pharmacology and Toxicology, School of Medicine, Medical Science CampusUniversity of Puerto Rico, PO Box 365067, San Juan, Puerto Rico 00936-5067
Received: February 13, 2024 | Published: February 26, 2024
Citation: Torres-Hernández BA, Serrano K, Santiago-Cruz Y, et al. Ethanolic valeriana officinalis extracts and valerenic acid delay pentylenetetrazole-induced seizures in adult zebrafish (Danio rerio): interactions with GABAa, glutamate, and adenosine receptors. Pharm Pharmacol Int J. 2024;12(1):24-28. DOI: 10.15406/ppij.2024.12.00427
Background: Anticonvulsant properties have been attributed to Valerian formulations and patients with epilepsy have reported using Valerian as complementary medicine. In zebrafish, Valerian ethanolic extracts (ValE) and valerenic acid (VA) have anticonvulsant properties.
Hypothesis/Purpose: We evaluated the possible role of GABAa, Adenosine, or Glutamate in the anticonvulsant properties of Valerenic acid (VA) and ethanolic Valerian extract (ValE) in zebrafish.
Study Design: Animals were pre-incubated with the selective antagonist and later with ethanolic Valerian extracts or valerenic acid. Untreated animals served as controls. The zebrafish were then exposed to pentylenetetrazole (3 mg/mL), and the latency to seizure-like behavior was recorded.
Results: GABAzine, a GABAa antagonist, reduces the anticonvulsant effects of ValE but not those of VA. On the other hand, A1 (DPCPX) and A2 (SCH-28261) antagonism altered the anticonvulsant effects of VA but not those of ValeE. The anticonvulsant effects of ValE are reduced by the antagonism of NMDA (D-AP5) and KA (UDP-301) but not by AMPA (NBQX). Metabotropic glutamate receptor (mGluR I/PHCCC, mGluR II/EGLU, mGluR III/CPPG) antagonists alter the effects of VA without altering ValE.
Conclusions: VA and ValE selectively interact with GABAa, Adenosine, and Glutamate receptors. Differences between the effects on the anticonvulsant effects of VA and ValE suggest that VA is not the only active constituent responsible for the ValE anticonvulsant activity.
Keywords: valerian, seizures, zebrafish, GABA, adenosine, glutamate
AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; CPPG, (RS)-α-Cyclopropyl-4-phosphonophenylglycine; DAP-5, D-(-)-2-Amino-5-phosphonopentanoic acid; DPCPX, 8-Cyclopentyl-1,3-dipropylxanthine; EGLU, (2S)-a-Ethylglutamic acid; Gabazine, 2-(3-Carboxypropyl)-3-amino-6-(4methoxyphenyl) pyridazinium bromide; GluR, glutamate receptors; iGluR, ionotropic glutamate receptors; KA, 2 carboxy 3 carboxymethyl4 isopropenylpyrrolidine; 2 carboxy 4 isopropenyl 3 pyrrolidineacetic acid; mGluR, metabotropic glutamate receptors; MK-801, (5S,10R)-(+)-5-Methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine maleate; NBQX, 2,3-Dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulfonamide disodium salt; NMDA, N-methyl-D-aspartate; PHCCC, N-Phenyl-7-(hydroxylamino)cyclopropa[b]chromen-1a-carboxamide; PTZ, Pentylenetetrazole; UBP-301, (αS)-α-Amino-3-[(4-carboxyphenyl)methyl]-3,4-dihydro-5-iodo-2,4-dioxo-1(2H)-pyrimidinepropanoic acid; SCH-58261, 2-(2-Furanyl)-7-(2-phenylethyl)-7H-pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidin-5-amine; VA, valerenic acid; ValE, valerian ethanolic extract
Valeriana officinalis formulations are sold to reduce anxiety and as a natural sleep aid and have been used in traditional medicine in Europe and Asia.1,2 Anticonvulsant properties have also been attributed to Valerian formulations.3–5 Furthermore, patients with epilepsy have reported using Valerian as complementary medicine.6,7
GABA, glutamate and adenosine play an important role in seizures and epilepsy.8-10 Valerian interacts with GABAa,11 glutamate receptors,12,13 serotonin,14 and adenosine receptors.15,16 Valerenic acid (VA) and Valerian ethanolic extracts (ValE) delay the onset of seizure in adult Danio rerio (zebrafish).17
However, the exact mechanism(s) mediating Valerian’s anticonvulsant effects are unclear. Thus, we examined their possible role in the the anticonvulsant properties of Valerenic acid (VA) and ethanolic Valerian extracts (ValE) are mediated thru interaction(s) with GABAa, adenosine, or glutamate receptors using the pentylenetetrazole-induced seizures in adult zebrafish.
Animals and Maintenance
Adult males and females, wild-type short-fin Danio rerio (zebrafish), 3–6 months old, and weighing 0.25 ± 0.04 g (mean ± SEM) were obtained from Caribe Fisheries Inc. (Lajas, Puerto Rico). Zebrafish were maintained in an aquarium with an automatic filtration system and covered with blue contact paper to reduce stress. An automatic feeder fed fish two times daily with Wardley Tropical Fish Premium Flakes Food. The zebrafish room was illuminated on a 14/10-hour light/dark cycle. Room temperature was maintained at 25° ± 1°C. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and approved by the Institutional Animal Care and Use Committee of the University of Puerto Rico, Medical Sciences Campus (Protocol number 3180110).
Chemicals
Valerenic acid (VA) with adjusted purity of 99.5% was purchased from ChromaDex® Reference Standards (Irvine, CA.) The valerenic acid stock solution was prepared as previously reported (12) by dissolving Valerenic acid powder in ethanol (70 %) and stored at −20°C for no more than two months. Dilutions were prepared with aquarium water to obtain the desired concentration of VA in the water. The following reagents were obtained from Tocris Bioscience (Ellisville, MO): N-Phenyl-7-(hydroxylamino)cyclopropa[b]chromen-1a-carboxamide (PHCCC), (2S)-a-Ethylglutamic acid (EGLU), (RS)-α-Cyclopropyl-4-phosphonophenylglycine (CPPG) and (5S,10R)-(+)-5-Methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine maleate (MK-801), all of which were prepared in aquarium water and (αS)-α-Amino-3-[(4-carboxyphenyl)methyl]-3,4-dihydro-5-iodo-2,4-dioxo-1(2H)-pyrimidinepropanoic acid (UBP-301) which was dissolved in Dimethyl sulfoxide (DMSO). All other antagonists were obtained from Ascent Scientific or ABCAM. 2,3-Dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulfonamide disodium salt (NBQX), D-(-)-2-Amino-5-phosphonopentanoic acid (DAP-5) were dissolved in aquarium water. 8-Cyclopentyl-1,3-dipropylxanthine (DPCPX) was dissolved in 70% ethanol; 2-(3-Carboxypropyl)-3-amino-6-(4methoxyphenyl) pyridazinium bromide (Gabazine) and 2-(2-Furanyl)-7-(2-phenylethyl)-7H-pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidin-5-amine (SCH-58261) were dissolved in DMSO. A stock solution of Pentylenetetrazole (PTZ, Sigma) was prepared with Milli-Q ultra-pure water and diluted with aquarium water to the desired concentration.
Valerian extract
Ethanolic Valerian extracts (ValE) were prepared from certified organically grown dry powdered roots (Lot 111H-OUP) obtained from Pacific Botanicals (LLC Grants Pass, Oregon). The extraction method was previously described.12 Briefly, dry Valerian powder was stirred in 70% ethanol in a covered beaker at room temperature for one hour with a final concentration of 150 mg/ml. Particles were removed by filtering through coarse filter paper. Extracts were prepared daily.
Pretreatment with antagonists
All experiments were performed between 8:00 and 17:00 with animals randomly selected (both sexes) from the housing tanks. Zebrafish were immersed in a 15-ml chamber (3.0 cm x 5.0 cm x 2.5 cm) containing the chosen treatment (antagonist or Val) dissolved in aquarium wate for one hour. Treatments with VA were also done by immersion, but the exposure time was limited to three minutes due to the toxicity of VA at higher concentrations.17
The possible interaction with the receptors was ascertained by treating animals with selective antagonists. The concentrations were chosen based on prior experiments performed in our laboratory.13,17 The concentrations were chosen based on prior experiments performed in our laboratory.13,17 The valeriana concentration of 37 μg/mg constitutes a compromise between the anticonvulsant effect and the excessive sedation. After one hour of pretreatment with a given antagonist, one-half of the animals were transferred to a chamber with ValE (1mg/ml) for one hour or VA (37 µg/ml) for three minutes (to avoid toxicity), then seizures were induced with PTZ (3 mg/mL). Our control groups were untreated animals, antagonists, VA, or Val-treated animals before exposure to PTZ. seizures. The ethanol and DMSO concentrations used as solvents did not affect the convulsion onset (data not shown).
PTZ-induced seizures experiments
Control groups and pretreated fish were transferred to a clear plastic tank (7.5 cm x 4.5 cm x 6.0 cm) containing PTZ (3 mg/ml; 21.7 mM) in aquarium water. Behavior was continuously monitored and recorded with a Panasonic SD Video Camera, Model # SDR-S26, until the convulsion onset (observed as uncoordinated jumping or clonic-like movements (convulsion), immediately followed by postural loss as previously defined12 or after a maximum of 5 minutes had elapsed. The convulsion progression was evaluated using the scale developed by our group. Briefly, when the fish were transferred to the tank, initially, they swam near the bottom (stage 1). As time passed, the fish had a rapid movement, hit the tank wall, and erratic and circular movement (stages 2-6) until they did a wild jump, had a clonic-like movement, and loss the posture (stage 7).14 The time of the convulsion onset was determined from addition of the fish to the PTZ solution until the first “seizure” (abrupt movements and loss of posture) determined from the recorded videos.
All PTZ exposure recordings always included untreated animals as internal positive controls. We named delayed convulsion onset when the animals had a convulsion onset significantly higher than the onset of the control animals, only exposed to PTZ.
Statistical analysis
Experiments were performed with 12-16 zebrafish per treatment. The latency (time to show clonic-like movement, stage 7) of each treated zebrafish was normalized against the latency of the animals exposed to PTZ alone. We exclude from the Analysis those animals which did not reach stage 7. Data are presented as Relative Time to the convulsion onset, the mean + standard error of the mean (SEM). Normalization and Analysis of variance (ANOVA) were calculated using GraphPad Prism software (La Jolla, CA (https://www.graphpad.com/). One-way ANOVA followed by Tukey posthoc test was performed, using a p < 0.05 level of significance.
Figure 1 shows that zebrafish pretreated with gabazine, a GABAa antagonist, (11.8 µg/ml) for one hour, followed by a three-minute exposure to VA did not display a seizure latency significantly different from that of VA alone. In contrast, GBZ preincubation significantly reduced the anticonvulsant effects of one hour ValE. The latency of each treated zebrafish was normalized against the latency mean of the zebrafish exposed to PTZ alone.
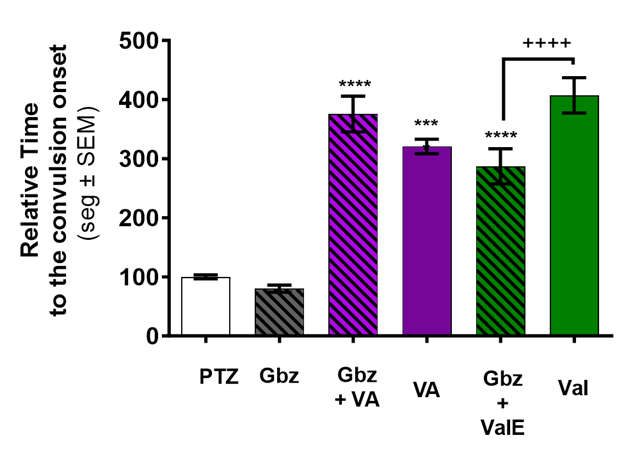
Figure 1 VA and ValE delayed convulsion-onset are modulated by pretreatment with a GABAa antagonist (gabazine). Onset to convulsion after PTZ exposure was measured in untreated animals (white bar), Valerenic acid only (VA, purple bar), or ethanolic Valerian extract only (ValE green bar) animals shown. Animals pretreated for one hour with gabazine (black striped bars) were then exposed to VA for 3 minutes (striped purple bar) or to ValE for one hour (striped green). The delayed convulsion-onset of VA was slightly potentiated. Delayed convulsion-onset by ValE treatment was significantly reduced in gabazine-pretreated animals. Treatments with the GABA antagonist alone did not have an effect. The latency of each treated zebrafish was normalized against the latency mean of the zebrafish exposed to PTZ alone. Data are presented as Mean ± SEM; n = 20-24 for PTZ and antagonist and n=12-15 for the other treatments. **** p < 0.0001vs PTZ, ++++ p < 0.0001 vs ValE.
Pretreatment with DPCPX, a selective A1 antagonist (0.54 µg/ml), significantly increases the seizure latency produced by VA (p <0.0001, (431.9 vs 322.2 respectively) shown in Figure 2A. In contrast, DPCPX pretreatment did not affect the delayed seizure latency produced by ValE. The convulsion onset by VA is reduced from a relative time of 313.6 to 251.8 (p< 0.05) by pretreatment with SCH-58261, an A2 antagonist (0.54 µg/ml). In contrast, the pretreatment with SCH-58261 does not significantly change the convulsion onset compared to ValE alone (300.9 vs 283.5 with ValE), as shown in Figure 2B.
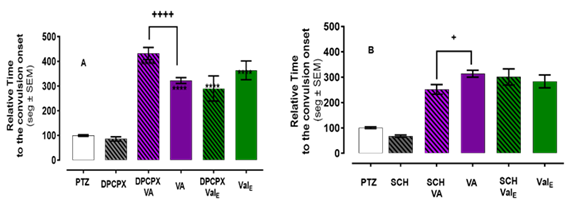
Figure 2 VA delayed convulsion-onset is modulated by pretreatment with by A1 (DPCPX) or A2 (SCH-58261) antagonist. Onset to convulsion after PTZ exposure was measured in untreated animals (white bar), Valerenic acid only (VA, purple bar), or ethanolic Valerian extract only (ValE green bar) animals shown. Animals pretreated for one hour with the antagonist (black striped bars) were then exposed to VA for 3 minutes (striped purple bar) or to ValE for one hour (striped green). (A) Pretreatment with an A1 antagonist, DPCPX (0.54µl/ml) increased the delayed convulsion onset of VA. (B) DPCPX 11.8 µg/ml, significantly delayed convulsion-onset. The increase observed in delayed convulsion-onset of VA and ValE after pretreatment with this concentration of DPCPX was not significant when compared with the zebrafish treated with DPCPX 11.8 µg/ml alone. (C) Pretreatment with A2 antagonist, SCH-58261 reduced the delayed convulsion-onset effect of VA. This concentration did not change the delayed convulsion onset of ValE to delay. The latency of each treated zebrafish was normalized against the latency mean of the zebrafish exposed to PTZ alone. Data are presented as Mean ± SEM; n = 20-24 for PTZ and antagonist and n=12-15 for the other treatments. **** p < 0.0001vs PTZ or ++ p < 0.01 or +++ p < 0.001.
The NMDA/Glu site antagonist, DAP-5 reduced the convulsion onset of ValE 260.1 vs 377.8 as compared to ValE alone (p <0.0001) (Figure 3A). However, DAP-5 did not affect the anticonvulsant effects of VA. Figure 3B shows that UBP-301, a kainate receptor antagonist, reduces the convulsion onset of ValE (201.6 vs 295.1, p=0.01), while no effect on the anticonvulsant effect of VA was observed. NBQX (AMPA receptor antagonist) pretreatment had no statistically significant effect on ValE or VA in the convulsion onset (Figure 3C).
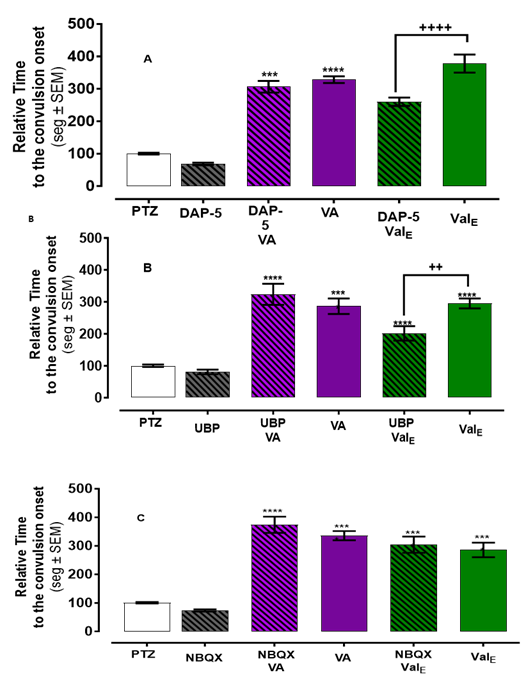
Figure 3 ValE effect modulated by iGluRs antagonist. Zebrafish pretreated with iGluRs antagonist and then exposed 3 minutes to VA had a similar time to convulsion onset. Pretreatment with (A) DAP-5 (NMDA antagonist)) or (B) UBP-301 (KA antagonist then one hour with ValE had a significant reduction in the relative time to convulsion onset compared with those treated with ValE alone. (C) Pretreatment with NBQX (AMPA antagonist) and then with ValE had similar convulsion onset. The antagonist alone did not delay the convulsion onset. The latency of each treated zebrafish was normalized against the latency mean of the zebrafish exposed to PTZ alone. The bar represents mean ± SEM n = 20-24 for PTZ and antagonist and n=12-15 for the other treatment. **** p < 0.0001vs PTZ or ++ p < 0.01 or ++++ p < 0.0001 vs ValE.
Pretreatment with PHCCC (mGluR group I antagonist) or EGLU (mGluR group II antagonist) before VA treatment significantly increased the anticonvulsant effect of VA (p <0.05), while not affecting ValE (11.8 µg/ml) shown in Figures 4A and 4B, respectively. In contrast, pretreatment with CPPG 11.8 µg/ml (mGluRs III antagonist) significantly (p < 0.001) reduced convulsion onset observed with VA (267.0 vs 365.01 with VA alone) but did not change the delay of the convulsion onset observed with ValE (Figure 4C).
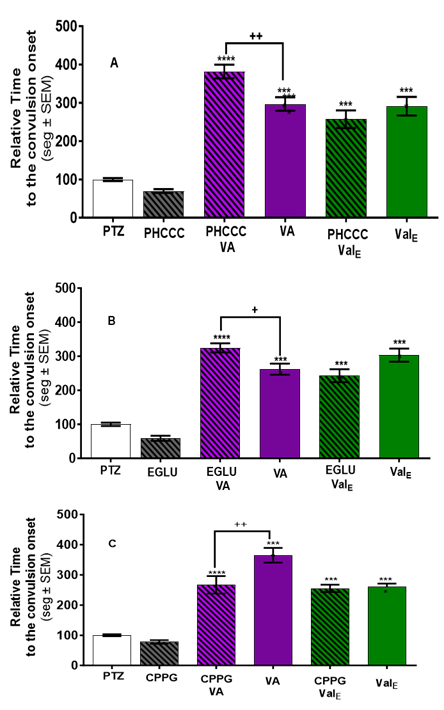
Figure 4 VA and ValE delayed convulsion-onset are modulated by pretreatment with mGluRs antagonist. Onset to convulsion after PTZ exposure was measured in untreated animals (white bar), Valerenic acid only (VA, purple bar), or ethanolic ValErian extract only (ValE green bar) animals are shown. Animals pretreated for one hour with the antagonist (black striped bars) were then exposed to VA for 3 minutes (striped purple bar) or to ValE for one hour (striped green). Delayed convulsion-onset produced by VA was significantly increased with (A) mGluRs I antagonist, PHCCC (11.8 µg/ml) pretreatment, and (B) mGluRs II antagonist, EGLU (11.8 µg/ml), but did not affect the delayed convulsion-onset produced by ValE. (C) mGluRs II antagonist, EGLU (1.8 µg/ml) reduced the delayed convulsion onset effect of ValE. (D) mGluRs III antagonist, CPPG reduced the delayed convulsion onset by VA but did not change the delayed convulsion onset by ValE. The latency of each treated zebrafish was normalized against the latency mean of the zebrafish exposed to PTZ alone. Data are presented as Mean ± SEM; n = 20-24 for PTZ and antagonist and n=12-15 for the other treatments. **** p < 0.0001vs PTZ; ++ p < 0.01 or ++++ p < 0.000 vs ValE.
Interactions of natural products with conventional drugs remain understudied, yet patients are willing to try natural products and dietary supplements.6,7 Adjuvant or complementary therapy could fill the gaps in the currently available antiseizure medications, such as their notorious side effects, resistance, and cost.18 Zebrafish constitute an excellent compromise between system complexity and practical simplicity and are useful for modeling, drug screening, and for mechanistic analysis of complex brain disorders. The zebrafish-PTZ model has been behaviorally and electrographically validated.19-23
More than 200 constituents have been identified in different Valerian root extract preparations.2,24 V. officinalis’ phytochemistry consists of esterified iridoid derivatives known as Valepotriates (e.g., valtrate, didrovaltrate, isovaleric acid), sesquiterpenes (e.g., Valerenic acid), flavonoids (e.g., linarin, apigenin), lignans (e.g., pinoresinol, hydroxypinoresinol), alkaloids (e.g., actinidine, Valerine), triterpenes (e.g., ursolic acid), monoterpenes (e.g., borneol, bornyl acetate).25
Valerenic acid, a “marker” compound for V. officinalis is credited with the pharmacological properties of Valerian extracts.1,2 The different effects of the antagonists on the VA vs. those of ValE suggest that other compounds may be exerting CNS effects. Moreover, other Valerian species with reduced Valerenic acid such as V. edulis or V. jatamansi, still have CNS active properties,25 support such hypothesis.
Activation of A1 receptors and endogenous adenosine have protective roles during seizures in rats and humans.27,28 The anticonvulsant effect of aqueous Valerian extract is reduced by adenosine antagonists in rats with kindled seizures,27 did not find significant differences in zebrafish injected with the selective antagonist DPCPX in doses lower than 10 mg//kg. In our experiments, pretreatment with DPCPX (0.54 µg/ml) had no effect, but potentiated the delayed convulsion onset effect of VA. On the other hand, DPCPX did not change the delayed convulsion onset produced by ValE in the zebrafish. Pretreatment with an A2 antagonist reduced the delayed convulsion onset produced by VA but did not change the delayed convulsion onset produced by ValE. Taken together, these results suggest interactions of VA, but not ValE, and A1 and A2 receptors.
VA, at high concentrations, acts as an allosteric modulator of the benzodiazepine site in the GABAa receptors.29 Moreover, activation of GABAa receptors by VA requires specific receptor conformation.29 The reduction by gabazine of the effects of ValE, but not those of VA, suggests that Valerian extract constituent(s), different from VA could bind to the GABAa to delay the convulsion onset in the zebrafish. Valerian extracts selectively interact with GluR.12,13 Our results show that KA and NMDA interactions are essential in the anticonvulsant mechanism of ValE but not of VA The opposite is observed with mGluR antagonists, as these agents alter the seizure latency of VA but not that of ValE.
The seizure-like behavior in these experiments was determined using animal behavior. While EEG analysis is possible (23), it is not well-suited for high throughput screening in adult zebrafish. In addition, a single antagonist dose allows for the identification of the involvement of a possible mechanism, without clarifying the nature of such interaction (i.e.. competitive antagonims vs partial agonism).
Our results show that VA and ValE selectively interact with GABAa, Adenosine, and Glutamate receptors. Differences between the effects on the anticonvulsant effects of VA and ValE suggest that VA is not the only active constituent responsible for the ValE anticonvulsant activity. Valerenic acid and Valerian extract interact with important pharmacological targets to control seizures. The properties to delay the convulsion onset of both Valerenic acid and Valerian ethanolic extract are mediated through multiple mechanisms, that involve GABAergic, adenosine, and/or glutamate synapses.
We appreciate the support of Dr. Rosa-Falero and Ms. Rigel Licier with some experiments. We appreciate Mr. Fernando Vera-Urbina’s conveying this manuscript to the graphical abstract. This work was supported in part by the Research Centers for Minority Institution (RCMI/NIH Grant no. G12 RR03051), Institutional Minority Biomedical Research Support (MBRS-RISE) Program at the University of Puerto Rico.
This research was part of the thesis project in partial fulfillment of the doctoral dissertation of Bianca A. Torres-Hernández. The authors have no conflict of interest. This manuscript is not under consideration by any journal.

©2024 Torres-Hernández, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.