eISSN: 2379-6367


Research Article Volume 11 Issue 4
1Life Sciences Department, College of Science and Technology, Al-Quds University, Palestine
2Faculty of Pharmacy, Al-Quds University, Palestine
Correspondence: Saleh Abu-Lafi, Faculty of Pharmacy, Al-Quds University, Abu-Dies, Palestine, Tel +972-2-2799360
Received: October 20, 2023 | Published: October 31, 2023
Citation: Akkawi M, Abu-Lafi S, Abu-Remeleh Q. Identification of key components in water extracts of tea (Camellia Sinensis) using UPLC-PDA-ESi-MS and evaluation of their possible antimalarial properties. Pharm Pharmacol Int J. 2023;11(4):137-146. DOI: 10.15406/ppij.2023.11.00412
This study used a semi-quantitative approach to examine how water extracted green and black tea (Camellia Sinensis) inhibits the formation of β-hematin in-vitro. LC-PDA-ESi-MS analysis of the tea extracts revealed that they contain many active catechins. When compared to the positive control, chloroquine, the results demonstrated that black water extracts had a considerable anti-malarial potential even at significantly lower dosages, while the green tea water extracts showed no effect. However, when a bicarbonate solution was used to create the green tea infusion, the result was similarly effective to the positive control. Additionally, there was no difference in β-hematin formation when the black tea was prepared at various time points (freshly prepared vs. 24-48 hours of incubation). The absence of time-dependent effects suggests that the in-vitro assay is unaffected by oxidation or any other changes that may have occurred in the extracts and solely reacts to stable active compounds or minerals that prevent β-hematin formation. The diverse combined catechins present in the tea extracts were efficient in forming a favorable complex with free heme, thereby preventing β-hematin formation, which could irreversibly harm the plasmodium parasite during the intraerythrocytic stage.
Keywords: green tea, black tea, Camellia Sinensis, malaria, hemozoin, β-hematin, catechins, UPLC-PDA-ESi-MS
ACTs, artemisinin-based combination therapies; UPLC, ultra performance liquid chromatography; PDA, photodiode array; ESi, electrospray ionization; MS, mass spectrometry; QDa, quadrupole mass analyzer detector; EGCG, epigallocatechin-3-gallate; EGC, epigallocatechin; ECG, epicatechin-3-gallate; EC, epicatechin; DMSO, dimethyl sulfoxide; NaHCO3, sodium bicarbonate; CQ, chloroquine diphosphate salt; ACN, Acetonitrile; FA, formic acid; H2O, water; NaOH, sodium hydroxide; A, Ahmad tea; T, Twining tea; F, Unpacked tea; R, Red rose tea; W, Wissotzky tea
Malaria is a dreadful disease that claims the lives of almost 500,000 people annually. The World Malaria Report 2022 estimates that there were 619,000 malaria-related fatalities in 2021, which is 9% more than in 2019 before the COVID-19 pandemic hit and 1% less than in 2020.1 31% of all deaths reported internationally were registered in Nigeria alone. 247 million cases of malaria were reported in the same year of 2021, largely affecting pregnant women and young children under the age of five.1
Malaria is an infection caused by single-celled Plasmodium parasites, which are transmitted to humans through the bite of an infected female Anopheles mosquito.2 During the bite, the pregnant mosquito injects its saliva to prevent the blood from clotting. The liver is the next stop for the plasmodium parasite once it enters the bloodstream, where they grow and mature over a period of days. Afterward, they move into the red blood cells, where they grow and eventually cause the cells to rupture, releasing other parasites into the bloodstream. When Plasmodium parasites break down hemoglobin, monomeric ferriprotoporphyrin (IX), a poisonous substance to the parasite, forms and accumulates.3-8
The parasite precludes the toxic monomer formation by forming a dimer, then polymerizing it into a harmless, insoluble polymer of dimers, called hemozoin. Identical to natural hemozoin is the synthetic β-hematin polymer which is synthesized from ferripro-toporphyrin (IX). Because the creation of hemozoin is essential for the parasite's survival, inhibiting β-hematin plays a vital key in the discovery of novel antimalarial drugs.9,10 Therefore, a candidate for an effective antimalarial drug works by preventing the formation of hemozoin, which eliminates the parasite.11-15
There are several effective treatments for malaria, including medications such as chloroquine, and artemisinin-based combination therapies (ACTs), etc. Recent years have seen an increase in the resistance of malaria parasites to widely used drugs. This resistance underlines the urgent need to develop novel, new antimalarial medications, especially from natural sources.16 Due to their varied chemical structures and functionalities, natural compounds, notably polyphenols, have drawn interest for their potential as antimalarial medicines.12 Polyphenols such as flavonoids and tannins, found in many plants, have shown promise antimalarial action by interfering with the parasite's life cycle and stifling its growth. These polyphenols have the benefit of being derived from nature, which may prevent the emergence of drug resistance, a critical obstacle in the treatment of malaria. There is currently continuing research into polyphenol-based antimalarials with the goal of better understanding their mechanisms of action and enhancing their efficacy. Utilizing the potential of natural polyphenols may provide creative approaches to the ongoing fight against malaria.11-15
Tea (Camellia Sinensis) belongs to Theaceae family and is considered one of the most consumed beverages worldwide. It is one of those rare beverages that are universally accepted by science for their numerous health benefits. Tea is loaded with bioactive poly-phenolic antioxidants such as catechins which act as free radical neutralizers protecting from cancer, and premature aging.17-23
Green tea contains various polyphenolic compounds, among which catechins are the most abundant. These catechins include epigallocatechin-3-gallate (EGCG), epigallocatechin (EGC), epicatechin-3-gallate (ECG), epicatechin (EC), and others. Among these, the EGCG is the most extensively studied major catechin present in green tea.24 The collective amount of catechins present in green tea contributes to about 85% of the total active metabolites found in it.24 However, most of the naturally occurring plants, including tea, still have relatively low levels of active ingredients. Hence, it is better to consume the entire water extract rather than isolate active components. This is because tea water extract contains many active ingredients that may work together in a synergistic manner.
Malaria primarily affects developing countries and kills a lot of people in vulnerable groups who cannot afford pricey antimalarial medications. We are encouraged to continue investigating the potential of various affordable water extracts such as green and black tea in reducing β-hematin formation in vitro. Moreover, the presumably active components in these water extracts were separated and identified chromatographically using UPLC connected to PDA detector and to a mass spectrometer (MS) in the electrospray ionization (ESi) mode.
Dimethyl sulfoxide (DMSO, 99.5%), sodium acetate (99%), and sodium bicarbonate (99.7-100.3%) were from Sigma-Aldrich. Positive control of chloroquine (CQ) diphosphate salt and hemin chloride were from Sigma. Glacial acetic acid was purchased from Fluka. The tea bags of Twinings and Ahmad green tea samples used in this investigation were products of England but purchased from a local shop. Three different types of black tea were used two of which were products of China. One of these is packed (red rose tea bag) while the other is not. The final sample comes from Wissotzky tea bags from Israel. All samples were kept at room temperature until use.
Preparation of green and black tea water extracts
Tea infusions were made by soaking 2 gr of tea bag material in 150 ml of distilled hot water for 20 minutes at 90°C. Afterward, it was allowed to cool to room temperature before filtering with MN615 Ø110mm filter paper. The concentrated extracts were evaporated using a rotary evaporator (IKA WEREKRV06-ML) between 60-70°C under reduced pressure, followed by freeze-drying (Labconco freeze drier) until a constant weight was attained. The final dry extracts were kept in a desiccator in opaque vials until UPLC-PDA-MS analysis.
In-vitro semi-quantitative assay for screening of antimalarial activity
The procedure implemented in the present research was the one established by Deharo et al.25 A brief description was as follows: a freshly prepared mixture containing 50μl of 0.5mg/ml hemin chloride (in DMSO), 100μl of 0.5M sodium acetate buffer at pH 4.4 and 50μl of the tested antimalarial drug solution or control, was incubated in a normal non-sterile 96-well flat-bottomed plate at 37ºC for 18-24 hours. The solutions must be added to the plate in this order. The plate was then centrifuged for 10 minutes at 4000 rpm. The supernatant was removed, and the pH of the reaction was measured. The final pH of the mixture should be between 5.0-5.2. The wells were washed with 200μl DMSO per well to remove free hemin chloride. The plate was centrifuged again, discharging the supernatant afterward. The β-hematin remaining was then dissolved in 200μl of 0.1M sodium hydroxide (NaOH) to form ferriprotoporphyrin IX that could be measured spectrophotometrically. The absorbance was then determined at 405nm using an ELISA reader. Milli-Q ultrapure water was used as a negative control, whereas chloroquine dissolved in ultrapure water was used as a positive control.
Chromatographic UPLC-PDA-MS analysis
Acquity UPLC H-Class system (Waters, USA) was used coupled to photodiode array detector (PDA) and quadrupole mass analyzer detector (QDa) and software of Empower. The column used was Acquity UPLC BEH C18 (50mm x 2.1mm I.D., 1.7μm) with Acquity BEH C18 1.7 μm guard column (Vanguard 2.1 x 5 mm, Waters, USA). Ultrapure water LC-MS grade (Milli-Q-Millipore) and Acetonitrile (ACN) Lichrosolv hypergrade (J.T. Baker), Formic acid (FA) HCOOH, LC-MS grade (Merck). The column and sample temperature were fixed at 35˚C and 25˚C respectively. The mobile phase consists of 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B). The gradient employed commences at 98% A, holding for two minutes before increasing to 100% B over the course of 12 minutes. The mixture is then kept at 100% B for an additional two minutes. The delay injection time was about 7 minutes to equilibrate the column. The flow rate used was 0.4 mL/min. The PDA range was from 210-500 nm. The MS ionization was in the negative and positive ESi modes, and the mass range was between 100-1200 Da.
In-vitro semi-quantitative method
Figure 1 shows a comparison of the in-vitro antimalarial effects of green and black tea extracts with chloroquine positive control and water negative control. Each absorption value is an average of 16 experiments. The absorption using the semi-quantitative method is inversely proportional to extract efficacy. Therefore, a lower absorption value implies greater extract efficacy. Results of the study indicate that all three local types of black tea extracts (designated as F, R and W) inhibited the production of β-hematin. However, the two green tea water extracts (designated as A and T) showed no effect.

Figure 1 Column chart shows the effectiveness of water infusions of black and green tea extracts compared to negative control (H2O) and positive control (CQ), showing absorption of dissolved β-hematin (alkaline hematin) at 405 nm using ELISA reader. The chart uses abbreviations to represent the types of tea used: A (Ahmad tea), T (Twining tea), F (unpacked tea), R (Red rose tea), and W (Wissotzky tea).
In contrast to green tea water extracts, which demonstrated no activity, the water extracts of black tea showed a potent inhibitory effect on β-hematin formation, even at significantly lower dosages (Figure 2). Figure 3 demonstrates that when black tea was prepared at different time points (freshly prepared vs 24-48 hours of incubation), no impact was observed on β-hematin formation. The lack of time-dependent effects indicates that the in-vitro assay only responds to stable molecules or minerals that inhibit β-hematin formation and is not influenced by oxidation or any other alterations that may have occurred in the tea extracts.26 Existing literature suggests that infusions of tea or coffee undergo oxidation and gradually lose their peroxides, which are crucial for eliminating parasites and microbes.26-28
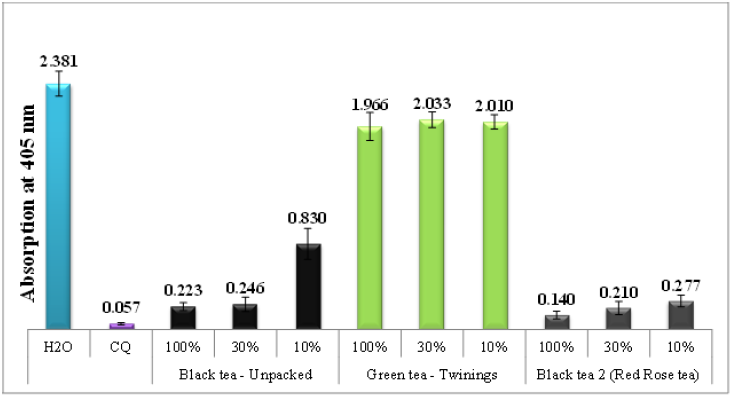
Figure 2 Column chart shows the effectiveness of different dilutions of black and green tea water extracts, as compared to negative and positive controls.

Figure 3 Column chart shows the impact of time on the efficacy of two types of black tea water infusions, F (unpacked) and W (Wissotzky), prepared at different time intervals, as compared to negative and positive controls.
Tea's pH may be impacted by the addition of sodium bicarbonate, creating a more basic or alkaline tea solution. This could then make some components in the tea more soluble, which would ultimately change the tea's color, flavor, and antimalarial characteristics. It's important to keep in mind that most people use tap water, which varies in alkalinity depending on where you live, to brew their tea. In Turkey, for example, local tea houses often use sodium bicarbonate to reduce the bitterness of the tea, enhance its color, and extract more infusion using fewer tea leaves.29 Studies show that adding sodium bicarbonate to tea might increase the body's absorption of magnesium and phosphorus, but moderation is crucial because too much sodium bicarbonate can have negative health effects. Figure 4 demonstrates that green tea infusion prepared using bicarbonate solution resulted in much better efficacy than those made with distilled water alone. It's important to note that the use of NaHCO3 can quicken the autooxidation of specific catechins, resulting in the browning of green tea infusion and giving it the appearance of black tea.29

Figure 4 Column chart shows the effectiveness of water extracts from green tea and NaHCO3-treated tea, in comparison to negative and positive controls.
In conclusion, the encouraging results suggest that the laboratory-based antimalarial activity of the crude black tea extract is of great significance. Due to tea's affordability, accessibility, and safety, this has the potential to assist financially challenged patients.
UPLC-PDA-ESi-MS analysis of tea extracts
Green tea and fermented oxidized black tea are the two main consumed types of tea.30,31 During the oxidation process that occurs in the fermentation of tea, monomeric flavonoids (catechins) found in the Camellia sinensis leaf are converted into polymeric theaflavin and thearubigin.30 In the UPLC-PDA-ESi-MS system, a sample of black tea was initially injected. The secondary metabolites in black tea have an approximate UV absorption at maximum wavelengths of 215, 257, 272, 315 and 351 nm. The UV maxima of these diverse chromophoric moieties of the polyphenols present in black tea are demonstrated in left side of figure 5. The UPLC-PDA chromatogram of the water extract of black tea at 272 nm is shown in figure 5. Figure 6 shows the TIC of the same sample using UPLC-MS-ESi in the negative mode. Most of the main peaks were seen in both detectors. To imitate herbal tea, only distilled water was utilized as a solvent. As a result of using water as an infusion solvent, only a moderate number of peaks were observed. Despite the small number of compounds found in the extract, the antimalarial effects were significant. Table 1 summarized the identity, retention time, molecular weight and the multiwavelength maxima of the separated polyphenolic compounds.
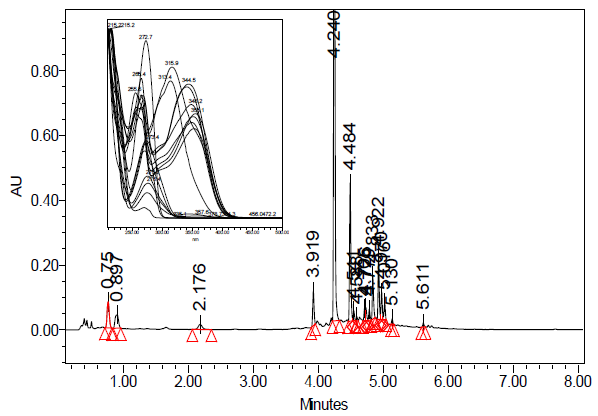
Figure 5 UPLC-PDA of freshwater extract of black tea (R) monitored at 272 nm. Overlaid PDA spectra of all peaks in water extract black tea superimposed within the range of 210-500 nm is in the left side.
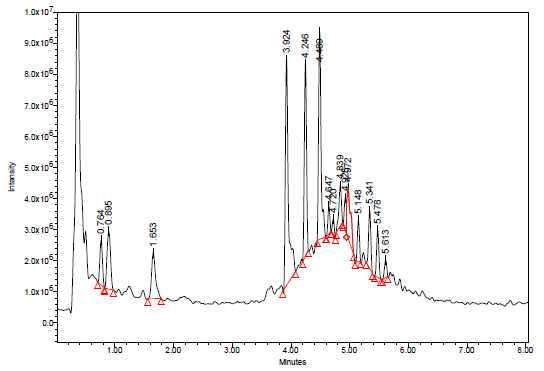
Figure 6 TIC of water extract of black tea using UPLC-MS-ESI in the negative mode. Full scan from 100-1200 Da.
Catechins are present in black and green tea, although the concentration and type of each individual catechin vary depending on many factors such as the type of tea, the processing methods used, and the brewing conditions, etc. An overlaid chromatogram of the four different samples used in this study is depicted below in figure 7. Figure 8 shows the mass spectrum of the main compounds present in black tea sample in the negative ESi mode. Figure 9 shows the chemical structures of the main constituents in black tea.

Figure 7 The overlaid UPLC-PDA profiles of the four tea samples. Red color (R), green color (W), blue color (T), and black color (F).

Figure 8 Mass spectrum of the main compounds present in black tea water extract in the negative ESi mode.
As can be seen in figures (5-8) and table 1, the naturally occurring polyphenolic compound, gallic acid eluted at 0.759 minutes and its UV-Vis spectrum showed a maximum absorption at 270.9 nm. Gallic acid mass spectrum (MS) in the negative ESi mode shows m/z of 169 amu corresponding to a typical deprotonated pseudo molecular ion [M-1]- of gallic acid (Figure 8). 5-O-galloylquinic acid was detected at 0.897 minutes with a deprotonated MS peak at 343 amu. Additionally, a dimer adduct peak of 5-O-galloylquinic acid at m/z 687 amu [2M-1]- was observed, which is commonly seen due to the high sample concentration injected. Gallocatechin (GC) had an elution time of 1.653 minutes, and its UV-Vis spectrum exhibited a peak at 268.4 nm. Additionally, its mass spectrometry (m/z) showed a value of 305 amu, corresponding to the characteristic deprotonated pseudo-molecular ions [M-1]-, consistent with gallocatechin's molecular mass of 306 amu. The peak at 2.17 minutes showed 181 amu represents a theobromine compound. Epigallocatechin (EGC), with an m/z of 305, was detected at 3.919 minutes, along with its dimer adduct [2M-1]- of 611 amu. The peak observed at 4.24 minutes corresponds to Procyanidin B1, which is a dimeric form of procyanidin (epicatechin-(4β→8)-catechin) with a m/z ratio of 577 amu (figure 9). Epigallocatechin gallate (EGCG) eluted at 4.484 minutes with m/z of 457, 915 (dimer). Similarly, Theaflavin displayed a mass of 563 amu, corresponding to a deprotonated molecular weight of 564 amu. Additionally, we observed the characteristic isomers of Quercetin-3-O-galactosyl-rhamnosyl-glucoside (with a molecular weight of 772 amu) eluting at 4.779 and 4.833 minutes, respectively. A subsequent group of peaks eluting between 4.922 and 5.611 minutes corresponded to compounds with mass-to-charge ratios (m/z) of 609, 441, 755, 593, and 1049 amu, respectively (table 1 and figure 9). We noticed that most of the abovementioned compounds were identified in tea using LC-MSMS.32-34
|
# |
Name |
*RT (mins) |
m/z [M-H]- |
UVmax (nm) |
|
1 |
Gallic acid |
0.759 |
169 |
270.9 |
|
2 |
5-O-galloylquinic acid |
0.897 |
343, 687 (dimer) |
273.4 |
|
3 |
Gallocatechin (GC) |
1.653 |
305, 611 (dimer) |
268.4 |
|
4 |
Theobromine |
2.17 |
181 |
272.7 |
|
5 |
Epigallocatechin (EGC) |
3.919 |
305, 611 (dimer) |
269.7 |
|
6 |
Procyanidin B1 |
4.24 |
577 |
227.7 |
|
7 |
Epigallocatechin gallate (EGCG) |
4.484 |
457, 915 (dimer) |
275.2 |
|
8 |
unknown |
4.541 |
337, 593 |
313.4 |
|
9 |
unknown |
4.588 |
457 |
274.6 |
|
10 |
Theaflavin |
4.705 |
563 |
276.8, 344.5 |
|
11 |
unknown |
4.726 |
563 |
269.1, 340.8 |
|
12 |
Quercetin-3-O-galactosyl-rhamnosyl-glucoside |
4.779 |
771 |
255.5, 355.1 |
|
13 |
Quercetin-3-O-glucosyl-rhamnosyl-glucoside |
4.833 |
771 |
256.2, 353.2 |
|
14 |
Quercetin-3-O-rutinoside |
4.922 |
609 |
257.4, 351.3 |
|
15 |
Epicatechin gallate (ECG) |
4.97 |
441 |
276.4 |
|
16 |
Kaempferol-3-O-glucosyl-rhamnosyl-glucoside |
5.016 |
755 |
265.4, 348.9 |
|
17 |
4’-O-Glucosylvitexin |
5.13 |
593 |
265.4, 348.2 |
|
18 |
Quercetin-3-(2G-p-coumtrans-3G)-2G-arabinosyl-3R- glucosylrutinoside |
5.611 |
1049 |
267.2, 315.9 |
Table 1 Compounds found in black tea (R) water infusion with their spectral characteristics
*RT: retention time in minutes, according to the UPLC-PDA profile
Catechins-heme complex formation
The key bioactive phytochemicals in tea, catechins, are distinguished by having numerous hydroxyl groups and, to a lesser extent, carbonyl, and occasionally carboxylic acid groups. These groups give catechins the ability to form many non-covalent catechin-heme complexes. It is believed that a stable and energetically advantageous combination between these catechins and ferriheme causes the suppression of β-hematin formation. Understanding the stability of these complexes is challenging due to the diverse hydroxyl groups present in many different catechins, adding to the complexity.
One could propose that the catechin-heme complex likely forms through two main interaction forces. The primary interaction likely occurs between the best fitted hydroxyl groups of catechins and the carbonyl group of unbound propionic carboxylate heme groups, involving hydrogen bonding. Additionally, hydrophobic π-stacking between the rings of catechins and the porphyrin rings of the heme moiety might also play a significant role in the underlying mechanism. By identifying the catechin-heme complex and shedding light on the complexities of their interaction, molecular modeling and appropriate docking experiments can be used to better understand the ease or difficulties surrounding such bindings.
According to our research, tea extracts contain catechins that have antiplasmodial properties, making them a promising option for treating malaria. These extracts' availability, cost, potency, safety, and ease of preparation enhance their potential as a remedy for malaria. These results provide opportunities for the creation of innovative and powerful antimalarial drugs. Black tea is preferable to green tea for best results, unless sodium bicarbonate is utilized with green tea to achieve successful results.
None.
The authors declare that there is no conflict of interest.

©2023 Akkawi, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.