eISSN: 2379-6367


Research Article Volume 10 Issue 5
1Laboratory of Immunology of Reproduction (LIR), Biochemistry and Pharmacy School, National University of Rosario (UNR), Argentina
2Research Council from National University of Rosario (CIUNR), Argentina
Correspondence: Adriana Brufman PhD, Agrelo 2101. S2005OQC-Rosario, National University of Rosario, Santa Fe, Argentina
Received: September 05, 2022 | Published: October 12, 2022
Citation: Brunori M, Raspo E, Grillo J, et al. Oxidative stress and iron metabolism in human sperm. Pharm Pharmacol Int J. 2022;10(5):185-188. DOI: 10.15406/ppij.2022.10.00383
Infertility is a global problem in couples. The decrease in sperm quality and function might be related to intrinsic and extrinsic factors, which ultimately converge on inducing oxidative stress (OS) in male gametes. Seminal plasma (SP) contains a large amount of antioxidants which protect sperm against OS. The iron in semen has a great influence on male fertility. Its transport, celluar uptake and storage depend on Transferrin (Tf), the cell membrane receptor for Transferrin (RTf) and Ferritin (FN), respectively. An isoform found in SP, testicular transferrin (TfT), is essential for spermatogenesis. The objective was to evaluate the antioxidant capacity of metalloproteins in SP involved in iron metabolism. A total of 328 semen samples from patients from the Urology Service of Eva Perón School Hospital (Granadero Baigorria) and Centenario Hospital (Rosario) (n=166) and healthy volunteers (n=162) were studied. Of these participants, 204 met the inclusion criteria. Semen samples were analyzed according to WHO criteria (2010-2021). Measurement of Fe and total proteins was carried out by colorimetric method, FN was measured by immunoturbidimetric method. ELISA technique was used for measuring the Tf concentration in SP. To measure OS we used 3 techniques: MOST test, (TBARs) technique and determination of total nitrite production by colorimetric method. Concentration of nitrites vs TfT showed an inverse relationship (R2=0,921; M= -0,7057; p<0,05). For abnormal MOST values (less than or equal to 0.39), the seminal Tf values were mostly below the mean value found in our population (5 mg/dL). An inverse relationship was seen between lipid peroxidation and TfT. (R2=0,4869; M=-0,0028). Our data suggest that TfT may fulfill an antioxidant role in the testis. It is necessary to study a greater number of patients and discriminate them by pathology in order to conclude which OS measurement technique is the most appropriate to assess male reproductive function.
Keywords: Seminal plasma, Reactive Oxygen Species (ROS), iron metabolism, male infertility
SP, seminal plasma; Tf, transferrin; Ir, iron; FN, ferritin; TfT, testicular transferrin; RTf, membrane receptor for transferrin ; TP, total protein; WHO, world health organization; ROS, rective oxygen species; RNS, reactive nitrogen species; SOD, superoxide dismutase; GPX, glutathione peroxidase; CAT, catalase; TBARS, thiobarbituric acid reactive substances; MDA, malondialdehyde; OS, oxidative stress; ON, overnight; ELISA, enzyme-linked immunosorbent assay; BSA, bovine serum albumin; RT, room temperature; H-HTF, human tubal fluid with HEPES; MOST, modified sperm stress
Infertility is a global problem affecting one-fifth of couples trying to conceive.1 Percentage of infertility due to male-related factors ranges from 20% to 70%,2 the decrease in sperm quality and function being the main cause.3 This decrease might be related to several intrinsic and extrinsic factors,4 which ultimately converge on inducing oxidative stress (OS) in male gametes.5 Antioxidants are compounds able to protect biological systems against reactions or processes that may produce a potentially harmful effect, such as reactive oxygen species (ROS) or reactive nitrogen species (RNS).6 Production of reactive oxygen species and other free radicals is a natural process of cell metabolism, however, exposure to contaminants, environment, lifestyle, and pathological situations, can generate an excess or accumulation of them (Figure 1).7
Accumulation of ROS can produce OS, which alters the spermatozoa and can cause membrane lipid peroxidation, DNA damage and cell apoptosis. ROS include radical and non-radical species. Similarly, the term RNS refers to a group of nitrogen-containing molecules with different chemical reactivity (Table 1).
Radicals |
Nonradicals |
Superoxide (O2- ) |
Hydrogen peroxide (H2O2) |
Hydroperoxyl (HOO-) |
Alkyl hydroperoxides (LOOH) |
Peroxyl (LOO-) |
Singlet oxygen (O2) |
Alkoxyl (LO-) |
Ozone (O3) |
Hydroxyl (-OH) |
Hypochlorous acid (HOCl) |
Nitric oxide (nitrogen monoxide; -NO) |
Peroxynitrite (ONOO-) |
Nitrogen dioxide (-NO2) |
|
Table 1 Radical and non radical species
To protect sperm from ROS-induced damage, SP contains a large number of antioxidants including enzymatic antioxidants (superoxide dismutase (SOD), glutathione peroxidase (GPX), and catalase (CAT)) and non-enzymatic antioxidants (calcium, iron, zinc, selenium) which protect sperm from DNA damage and lipid peroxidation.8 Iron in semen has a great influence on male fertility. It is essential for spermatogenesis and numerous functions of the sperm, participates in oxygenation and reduction processes and is essential to many enzymes and metalloproteins. This transition metal is involved in a variety of physiological, toxicological and pathological processes, due to its ability to undergo changes in its oxidation state, since its electronic configuration allows the generation of stable ferrous (Fe2+) or ferric (Fe3+) ions.
Transport, cellular uptake, and storage of iron in a useful and nontoxic state depends on the soluble iron transport protein, Transferrin (Tf), the cellular membrane receptor for Transferrin, and the storage cytoplasmatic protein, Ferritin. An isoform of Tf, testicular transferrin (TfT), is found in seminal plasma and is essential for spermatogenesis.9
To evaluate the antioxidant capacity of metalloproteins in seminal plasma involved in iron metabolism.
A total of 328 semen samples from patients from the Urology Service of Eva Perón School Hospital (Granadero Baigorria) and Centenario Hospital (Rosario) (n=166) and healthy volunteers (n=162) (age: 36.55±10.44) were studied from October 2019 to February 2022. Of these, 204 were selected that met the inclusion criteria. Samples were obtained with prior informed consent of the patients and controls, along with the treating professionals’authorization and endorsement according to Helsinki declaration. The framework project of this work has the approval of the bioethics committee of the School of Biochemical and Pharmaceutical Sciences, National University of Rosario, File 6060/368. Res CD 592/2017. Patients received written instructions for the semen collection. Low-volume samples (<1ml) and patients with clinical conditions that could affect Tf and FN levels in blood plasma, such as acute or chronic liver disease, neoplasia, clinical/laboratory signs of acute or chronic infection/inflammation, hepatitis viruses (A, B or C) infections, leukocytosis, fever, hypoproteinemia and diseases of iron metabolism were excluded.
Processing of semen samples
Semen samples, after liquefaction (30-60 minutes from collection), were analyzed according to the recommendations of the World Health Organization10,11 and evaluated using functional tests. Subsequently, an aliquot of 500μl was taken for swim-up technique. The rest of the semen sample was centrifuged for 30 minutes at a speed of 5,000 rpm and the resulting supernatant (the seminal plasma) was placed in a 1.5 ml eppendorf tube, to be centrifuged again to eliminate the remaining cells, and stored at -20ºC for later analysis.
Biochemical determinations on seminal plasma
Determination of Fe and total protein concentration was carried out by colorimetric method, FN was measured by immunoturbidimetric method. For these determinations, Cobas C501® analyzer (Roche Diagnostics) was used. All reagents and controls were from Roche-Diagnostics®.
ELISA technique was used for measuring the Tf concentration in SP
Seminal TF concentration was measured by sandwich enzyme-linked immunosorbent assay (ELISA) developed in our laboratory. Each sample was analyzed by triplicate. Well polystyrene strip plates (Nunc, Immuno MaxiSorp, U16) were coated with mouse monoclonal antibody [HTF-14] to Transferrin (Abcam–ab769) in 0.05M carbonate-bicarbonate coating buffer, pH = 9.6, overnight (ON) at 4°C. Next, wells were washed four times with 0.05% v/v Tween 20 in PBS, using a microplate washer (Vacuum System, Argentina). Wells were blocked by adding 3% w/v bovine serum albumin (BSA) in PBS during 2 h at room temperature (RT). Purified human TF (Sigma-Aldrich Inc.-Cas. No: 11096-37-0) was used as standard at concentrations from 0,1 mg/dl a 20 mg/dl. 50μl of either standard solution or samples were added to the wells and incubated for 1 h at RT. After washing, 50μl of polyclonal biotinilated anti-Tf antibody (Abcam-ab 19178) in 1% w/v BSA were added to the wells, and incubation was performed during 2 h at RT followed by washing with wash buffer, unbound conjugates are washed away.
Streptavidin-Peroxidase Conjugate is added and then, bound antibody was detected by incubation with horseradish peroxidase-streptavidin conjugate during 1 h at RT, followed by addition of a chromogenic substrate (3,3’,5,5’-tetramethylbenzidine) and H2O2 (Biopur®) during 10 min. The reaction was stopped with 1N H2SO4 and colored signal was quantified in a microplate autoreader (Mindray MR-96A, China) at 492 nm. A calibration curve was performed relating optical density vs. standard concentration. Tf concentration was finally expressed as mg of TF /dL. Detection limits were 0,3 mg/dL for transferrin; intra-assay and inter-assay coefficients of variations was 4.9% and 9.4%.
Swim-up technique
500ul of liquefied semen was carefully layered at the bottom of the tube which contains H-HTF medium (Human Tubal Fluid with HEPES) supplemented with 0.5% albumin (Irvine Scientific, CA) and it was left to incubate for 1hour at 37°C in 45o angle. The active and motile spermatozoa will move from the sample to the transparent medium, which is aspirated, obtaining the desired spermatozoa.12
To measure OS we use 3 techniques, to evaluate lipid peroxidation in spermatic membrane and to measure ROS and RNS in SP.
MOST (Modified Sperm Stress): Lipid peroxidation in spermatic membrane
MOST test (final translational mobility/initial translational mobility) evaluates loss of mobility in the spermatozoa after being incubated for 4 hours in a water bath at 40°C. The objective is to produce forced lipid peroxidation in sperm cells.13 To carry out this technique, the aforementioned swim-up technique was first performed. 10μL of the upper layer (where the capacitated sperm are found) were taken and the percentage of progressively motile sperm (initial motility) was determined. Right after, 200μL of the upper layer were taken and incubated for 4 hours at 40ºC in a water bath. Within 200μL, 10μL were taken and the percentage of progressively motile spermatozoa (final motility) was also determined.
MOST value calculated as follows
MOST: Final motility/Initial motility ≥ 0.44
Thiobarbituric acid reactive substances
Malondialdehyde (MDA) is the end product of fatty acid peroxidation and a marker of free radical activity. For the measurement of lipid peroxidation in SP, thiobarbituric acid (TBA) method was used.14 It is the most widely used method to determine lipid oxidation and, therefore, oxidative stress. TBA-reactive substances (TBARS) create a red complex, with an absorbance peak at 532 nm, as a result of the MDA with two TBA molecules reaction.
750µL of TBA agent (Merck Millipore) (0.8 g of 2-TBA dissolved in 80 ml of distilled water with 0.5 ml of NaOH) were added to 100µL of SP, and hydrochloric acid was added to adjust the pH. between 3-3.5. Samples were heated for 1 hour at 95°C in a dry bath (Thermo Scientific™), allowed to cool down to stop the reaction, and then centrifuged for 10 minutes at 800g. The color reaction was measured spectrophotometrically at 532nm on the supernatant. Absorbance measurements were performed on a Jenway 6705 spectrophotometer, Dunmow Essex. MDA concentration (measured as thiobarbituric acid reactive substances, TBAR) was calculated as nmol/L). In order to calculate the concentration of MDA from the absorbance values obtained, a calibration curve was made using a control of known concentration (MDA-Merck). The control was diluted to final concentrations of 1 nmol/L, 0.8 nmol/L, 0.5 nmol/L and 0.4 nmol/L.
Total nitrite production
Nitric oxide (NO) is a free radical which, in high concentrations, has detrimental effects on cells. NO levels in SP can be used as a marker of oxidative stress.15 NO measurement was performed by determining the total amount of nitrites (NO2−), which are the stable products of NO metabolism in seminal fluid. Griess reagent (aqueous solution of 1% sulfanilamide and 0.1% naphthylene ethylene diamide in H3PO4), which forms a stable chromophore with NO2− and absorbs at 546 nm, was used. 900μL of distilled water, 40μL of α-naphthylanim and 40μL of sulfanilic acid were added to 100μL of SP. Absorbance was read after 5 and 10 minutes; using a control solution of 0.05 ppm of NO- [NO2Na (Merk-Millipore) Cat 7632 in bidistilled water]. Absorbance measurements were performed on a Jenway 6705 spectrophotometer, Dunmow Essex. The nitrite concentration was calculated as mg/L.
Statistical analysis
To verify a possible correlation between the variables, a least squares regression line (r) was used. For comparison of Tf concentrations, a categorization into groups was carried out. For database, R Commander® and Graphpad Prism 9.2.0 were applied. Statistical significance was considered when p<0.05.
Nitrites
Figure 2 shows the values of Absorbance, NO2-chromophore concentration (ug/mL) and seminal Tf (mg/dL) for 112 samples, using a 0.05 ppm NO- solution as a control.
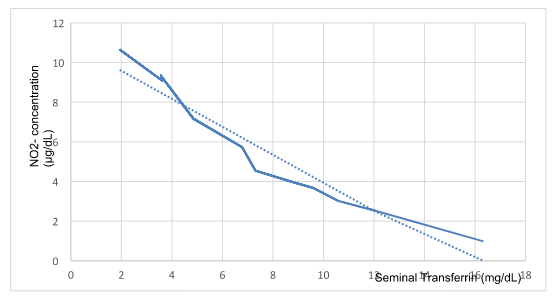
Figure 2 Nitrites vs seminal transferrin.
Concentration of Nitrites and seminal Tf showed an inverse relationship (R2=0,921; M= -0,7057; p0,05).
MOST
Figure 3 shows that for abnormal MOST values (less than or equal to 0.39), the seminal Tf values are mostly below the mean value found in our population (5 mg/dL).
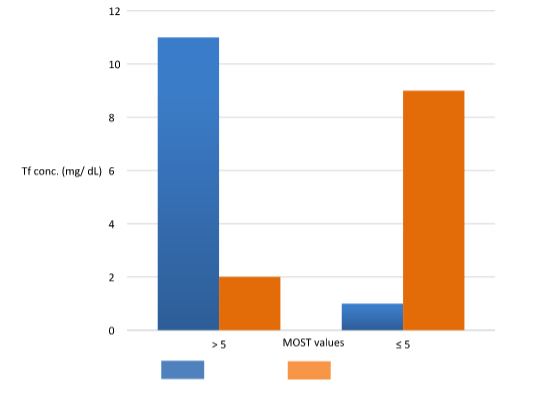
Figure 3 MOST values (Modified sperm stress) vs mean seminal Tf value.
MOST in seminal samples (n=118) with Transferrin values in SP greater than the mean (5 mg/dL) and values less than or equal to the mean. Blue: normal MOST test (>0.39). Orange: altered MOST test (≤ 0.39).
TBARs
The calibration curve was: [MDA] = 1,1385 x Abs535nm + 0,3167, R2 = 0.9712.
Figure 4 shows the absorbance’s corresponding to the MDA values by the T-Bars method compared to the seminal Tf concentration of the samples studied (n= 204).
ROS promote the emergence and development of diseases such as cancer, diabetes, atherosclerosis, neurodegenerative disorders and aging.16 In addition, they can trigger the formation of genotoxic and mutagenic products that would increase the risk of disease in the offspring.17
In testis, OS interferes with sperm capacitation and can cause damage to both sperm membrane and DNA, affecting its potential to fertilize the egg and generate a healthy embryo. ROS are produced primarily by abnormal and immature leukocytes in SP and are a natural product of oxidative metabolic pathways. Small amounts are required to ensure cellular physiological functions, including spermatogenesis, capacitation, and the acrosome reaction.18 The imbalance of ROS production and antioxidants production causes oxidative stress which could negatively affect fertility.19 Therefore, accurate measurement of ROS provides a vital tool in the initial evaluation and follow-up of infertile patients.
Depending on the assay methodology used, recent literature suggests that 30% to 80% of infertile men have elevated levels of seminal ROS, a potentially treatable condition. MOST has been proposed as a predictor of sperm fertilization capacity and has been described as an effective technique to assess membrane lipid peroxidation. In our study, we observed that all samples with high peroxidation (MOST values greater than 39), regardless of sperm concentration, had a low concentration of Tf. We also observed that low concentrations of Tf in SP corresponded to higher values of ROS. It could be considered that the Fe-Tf system contributes to maintaining the redox balance in the testis.
Based on our results, we propose to integrate Transferrin measurement in SP (as an additional measurement of ROS) into the study of infertile couples, particularly those with idiopathic infertility. It is necessary to study a greater number of patients and to discriminate them by pathology in order to conclude which ROS measurement technique is the most appropriate to assess male reproductive function.
Research and Development Project, UNR. “Characterization of Iron Transporting Metalloproteins in Seminal Plasma and their Possible Function as Regulatory Molecules of Sperm Metabolism” (BIO595) (Res.CS N°650/2017).
To the patients who participated in the project. To urologist Esteban Streiger (MD), for his collaboration in data collection. To Chemist Ricardo Botta for his advice in the preparation of reagents and to Lic. Alejandra Olmos for her assistance in the statistical processing.
Authors declare that there is no conflict of interest.

©2022 Brunori, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.