eISSN: 2379-6367


Research Article Volume 10 Issue 6
Department of Clinical and Biological Sciences, Laboratory of Clinical Pharmacology “Franco Ghezzo”, University of Turin, Italy
Correspondence: Sarah Allegra (BSc, MSc, PhD), Department of Clinical and Biological Sciences, Clinical Pharmacology Service Franco Ghezzo, University of Turin, San Luigi Gonzaga Hospital - Regione Gonzole 10, 10043, Orbassano (TO), Italy, Tel +39 011 6705442
Received: October 20, 2022 | Published: November 4, 2022
Citation: Allegra S, Chiara F, De Francia S. Posaconazole plasma concentrations in children and adolescent. Pharm Pharmacol Int J. 2022;10(6):196-199. DOI: 10.15406/ppij.2022.10.00385
Although posaconazole is licensed only for use in children over 13 years of age, it has been also used in younger children for invasive fungal infections prophylaxis. Its variable and unpredictable oral bioavailability and its saturable absorption, make it difficult to determine the optimal dosing regimen. Hence, therapeutic drug monitoring could improve drug treatment success and safety. Here we described posaconazole pharmacokinetics in paediatrics and we evaluated the role of therapeutic drug monitoring for therapy and prophylaxis. A chromatographic method was used to determine posaconazole exposure in plasma. Pearson and Mann-Whitney U test were used. A high inter-individual variability was shown; an inverse and significant correlation was found among drug concentration and its dose. Moreover, in adolescent, we observed an inverse correlation between drug exposure and age and the influence of sex of PSC levels. These results highlights that strict therapeutic drug monitoring is required to achieved an adequate target posaconazole serum levels.
Keywords: therapeutic drug monitoring; triazoles, HPLC, invasive fungal infections, paediatrics
Invasive fungal infections (IFIs) are still a leading cause of morbidity and mortality in immunocompromised and severely ill hospitalized patients. In paediatrics, the incidence of IFIs is higher than the 10%, considering patients with acute myeloid leukaemia or recurrent acute leukaemia and in patients who undergo hematopoietic stem cell transplantations.1
Azoles are the first choice for IFIs prevention and treatment. For a long time, the available antifungal drug was amphotericin B deoxycholate and 5-fluorocytosine. Today, new antifungal agents, with different pharmacologic properties, including the lipid formulations of amphotericin B, are available: fluconazole, itraconazole (first generation triazoles), voriconazole, posaconazole, isavuconazole (second generation triazoles), anidulafungin, caspofungin, micafungin (echinocandins).2
Posaconazole (PSC; Noxafil®) is a second-generation triazole, similar to itraconazole, with a broad spectrum of activity. In Europe, PSC is licensed only for use in children over 13 years old, although it has been used in younger children for the treatment of severe IFIs as off-label prescription. A PSC linear pharmacokinetics has been reported, considering a daily dose up to 800 mg; further dose increases do not determine a proportional increase in drug plasma concentration. PSC time to reach the maximum serum concentration is 5 hours and it reaches the half-life after 34 hours (1 week), thus repeated dosing results in a relatively constant drug plasma concentration at steady state.3 The drug is primarily metabolized by glucuronidation rather than oxidation; it is a cytochrome (CYP) 3A4 activity inhibitor.4 PSC is available as a 40-mg/mL oral suspension, as delayed-release tablets and as an intravenous infusion. The first approved PSC formulation was the oral suspension; however, its absorption is highly variable and it depends on gastrointestinal conditions (reduced gastric acidity): the consumption of high fat meals prior to drug administration could improve PSC uptake.5 In addition to the oral suspension formulation, a new formulation of a delayed release tablet was approved, providing a consistent absorption independent of the gastrointestinal conditions or concomitant medication and thus with an improved drug bioavailability.6 The oral suspension should be administered for prophylaxis three times daily in order to reach the required PSC plasma concentration.5 Instead, the recommended PSC dose of tablets for prophylaxis or treatment, for adolescents of 13 years of age and older, is a once daily dose of 300mg, after an initial dose of 300mg twice daily on the first day.7 However, attaining target PSC concentrations in children is challenging: the variability of the dose required to obtain the adequate drug serum concentrations is the result of the unpredictable PSC bioavailability in these patients.8-10
Considering paediatrics, not many data are available in literature, and a large part of them are based on adult knowledge. Moreover, PSC pharmacokinetics in adolescents seem comparable to those reported for adults.11 On the contrary, to obtain adequate drug concentration is difficult in young patients and a high variability in dose required has been observed.12 To date, no guidelines for children under 13 years of age are available.13
The aim of our study was to report PSC pharmacokinetics description in paediatrics patients and to evaluate the utility of plasma concentration drug monitoring in therapy and prophylaxis.
Plasma samples were collected at the Clinical Pharmacology Service "Franco Ghezzo" (Department of Clinical and Biological Sciences, University of Turin, S. Luigi Gonzaga Hospital) from different Hospitals in Piedmont (Italy). Inclusion criteria were: age below 18 years old, diagnoses of IFIs and oral tablet or suspension PSC treatment or IFI prophylaxis with oral tablet or suspension PSC, with an adherence of 90%. Patients reported allergy or intolerance to PSC, HIV infection, severe malnutrition, liver cirrhosis, chronic renal failure (with estimated creatinine clearance<60 mL/min) were excluded. Study protocol ("PkPG_J02AC Studio retrospettivo per la valutazione e farmacocinetica e farmaco-genetica della terapia antimicotica con farmaci triazolici”) was approved by the local Ethics Committee. A written informed consent for the study was obtained from each subject, signed by natural/biological father or mother of a child with full parental legal rights. The study was performed in accordance with the Helsinki Declaration of 1975.
For all the enrolled patients, following data were collected: gender, age, body mass index (BMI), ethnicity and PSC dose.
Plasma PSC concentration were determined from blood samples obtained at the end of dosing interval, before the next drug dose intake. Patient blood samples were collected in the lithium-heparin tube and centrifuged at 1500 rpm for 10 minutes at 4°C. PSC quantification was performed by a validated High Performance Liquid Chromatography method coupled with UV detection (HPLC-UV).14 Internal standard quantification was used, fitted with linear regression. For descriptive statistics, continuous and non-normal variables were summarized as mean, standard deviation (SD), median values (considering all the enrolled patients) and the interquartile range (IQR, quartile 1; quartile 3) was calculated to measure the statistical dispersion of the data; categorical variables were described as frequency and percentage. All the variables were tested for normality with the Shapiro-Wilk test. The correspondence of each parameter was evaluated with a normal or non-normal distribution, through the Kolmogorov-Smirnov test. Non-normal variables were described by median values (plasma concentration). Mann-Whitney test has been used to compare plasma concentration and sex (level of statistical significance p-value < 0.05). Any predictive power of the considered variables was finally evaluated through univariate and multivariate linear (β coefficient) for continuous variables, and logistic (OR, odd ratio), considering therapeutic range, regression analyses (IC, interval of confidence at 95%). Factors with p-value < 0.2 in univariate analysis were considered in multivariate analysis (level of statistical significance p-value < 0.05). All tests were performed with IBM SPSS Statistics 22.0 per Windows (Chicago, Illinois, USA).
Twenty-six paediatric patients (17 males, 65.4%), treated with PSC, were enrolled. A great percentage of them (N=20; 76.9%) were Caucasians. Twenty (76.9%) received PSC antifungal prophylaxis; considering all the enrolled patients, route of administration was oral.
Mean, SD, median and IQR values for age, weight, BMI, creatinine serum levels, PSC drug dose and PSC plasma concentration were resumed and compared in Table 1; there were no statistically significant differences in terms of baseline characteristics.
Variable |
N=26 |
N=24 |
||||||
Mean |
Standard Deviation |
Median |
IQR |
Mean |
Standard Deviation |
Median |
IQR |
|
Age (years) |
15.5 |
3.409 |
17 |
13.75-18.00 |
15.89 |
2.105 |
17 |
14.00-18.00 |
Weight (Kg) |
58.31 |
17.748 |
57.85 |
50.00-67.00 |
62.57 |
14.741 |
60 |
54.00-67.00 |
BMI (Kg/m2) |
20.64 |
3.145 |
20.68 |
18.95-23.29 |
21.56 |
2.279 |
21.33 |
19.88-23.44 |
Creatinine serum levels (mg/dL) |
0.69 |
0.392 |
0.71 |
0.41-0.88 |
0.72 |
0.399 |
0.76 |
0.41-0.92 |
PSC Ctrough(ng/mL) |
576.62 |
559.618 |
479.5 |
143.25-756.50 |
633.05 |
607.754 |
527 |
226.00-750.00 |
Table 1 Mean, standard deviation, median and interquartile range for age, weight, body mass index, creatinine serum levels and posaconazole plasma concentrations considering all the enrolled patients (N=26) and only adolescent (age ≥13 years old; N=24)
PSC, posaconazole; N, number; IQR, interquartile range; BMI, body mass index; Ctrough, concentration at the end of dosing interval
The drug dosages were: 100 mg three times daily (N=2; 7.7%), 200 mg twice daily (N=3; 11.5%), 200 mg three times daily (N=14; 53.8%) and 400 mg three times daily (N=7; 26.9%).
Based on published PSC through levels cut-offs,15 we observed that 6 patients (100%) showed sub-optimal exposure evaluating those receiving PSC treatment; instead, for prophylaxis, 6 patients (30%) showed trough levels lower than the efficacy cut-off and14 (70%) had concentrations included in the efficacy range.
A high interindividual variability was found between PSC Ctrough concentrations: the median value was 479.50 ng/mL and the IQR range was 143.25ng/mL and 756.50 ng/mL. Pearson correlation test showed an inverse and statistically significant correlation among PSC Ctrough and drug dose (r=-0.494; p=0.010; Figure 1).
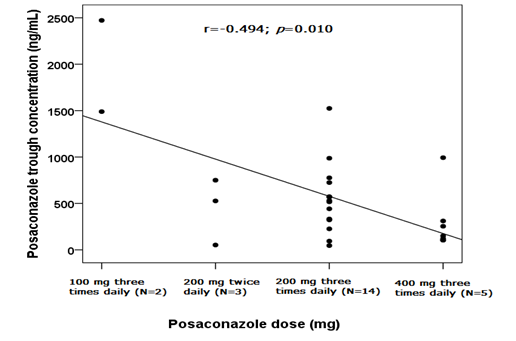
Figure 1 Scatter plot and linear correlation between posaconazole trough concentration (ng/mL) (y) and drug dose (mg) (x), considering all the 26 patients. The Pearson correlation coefficient (r) was -0.494 and the p-value was 0.010. Trend line and interval of confidence at 95% were shown.
Considering adolescent, twenty-four patients (15 males, 62.5%) were analysed. Twenty of them were Caucasians (83.3%). Six (25.0%) received PSC antifungal treatment. Mean, SD, median and IQR values for age, weight, BMI, creatinine serum levels, PSC drug dose and PSC plasma concentration were resumed and compared in Table 1; there were no statistically significant differences in terms of baseline characteristics.
The drug dosages were: 100 mg three times daily (N=2; 8.3%), 200 mg twice daily (N=3; 12.5%), 200 mg three times daily (N=14; 58.3%) and 400 mg three times daily (N=5; 20.8%).
Considering the efficacy cut-offs,15 in 6 patients (100%) receiving antifungal treatment we reported PSC concentrations under the lower efficacy threshold. Evaluating prophylaxis, 4 patients (22.2%) showed a sub-optimal drug exposure and 14 (77.8%) had concentrations included in the efficacy range. Also with this case selection, we observed a high interindividual variability: median PSC Ctrough was 527.00 ng/mL (IQR:226.00-750.00 ng/mL). Performing the Pearson correlation test, we observed significant correlations between PSC Ctrough and drug dose (r=-0.574; p=0.003; Figure 2) and PSC Ctrough and age (r=-0.492; p=0.015; Figure 3). The PSC levels resulted significantly influenced by sex since females (N=9) had higher values than males (N=15): 598 ng/mL (IQR 187-993 ng/mL) vs. 517 μg/mL (IQR 226-661 ng/mL), p=0.012, (Figure 4).
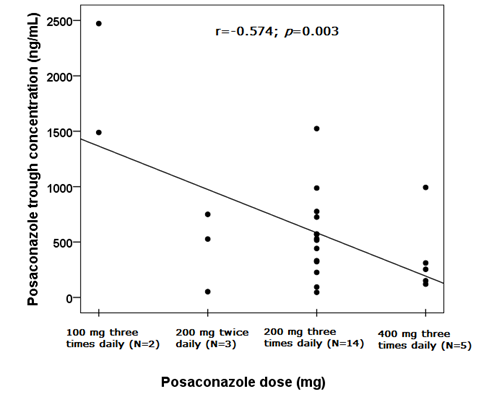
Figure 2 Scatter plot and linear correlation between posaconazole trough concentration (ng/mL) (y) and drug dose (mg) (x), considering adolescent patients (N=24) with oral route of administration. The Pearson correlation coefficient (r) was -0.574 and the p-value was 0.003. Trend line was shown.
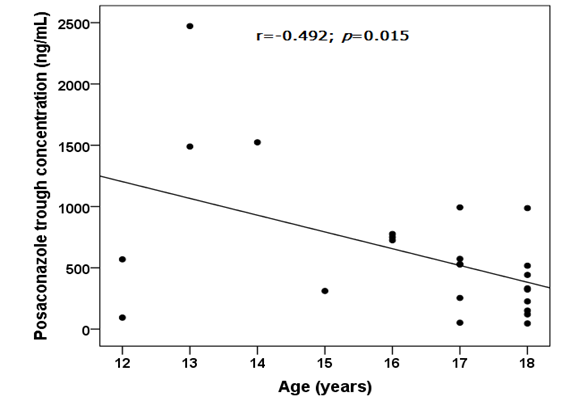
Figure 3 Scatter plot and linear correlation between posaconazole trough concentration (ng/mL) (y) and age (years) (x), considering adolescent patients (N=24) with oral route of administration. The Pearson correlation coefficient (r) was -0.5492 and the p-value was 0.015. Trend line was shown.
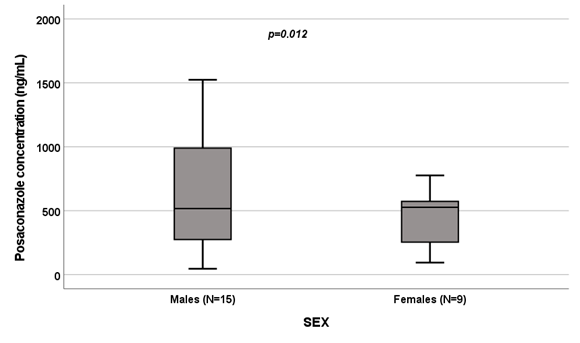
Figure 4 Influence of gender on posaconazole levels [ng/mL].
Box plot of gender influence on posaconazole levels (ng/mL); boxes and black lines in boxes represent respectively interquartile ranges (IQR) and median values; open dots and stars represent outlier values. Median values (horizontal line), IQR (bars), patient values (black square), highest and lowest value (whiskers) and p value are shown.
The IFIs mortality and morbidity increase over the years even the new better and faster diagnostic methods and the possibility of a wide range of antifungal treatments. IFIs are a major cause of life-threatening diseases in immunocompromised patients, including cancer patients receiving chemotherapy, hematopoietic stem cell and solid organ transplant recipients, HIV positive patients, those receiving invasive clinical procedures or patients hospitalized in intensive care units.16
PSC acts by inhibiting lanosterol-14-α-demethylase (CYP51), blocking the synthesis of ergosterol, causing cell membrane stability impairment, resulting in fungistatic or fungicidal effects.17 It is an antifungal drug frequently used prophylactically in children due to its broad spectrum of activity, including also Aspergillus spp. and Zygomycetes. Moreover, it shows a good efficacy and safety profiles both in adults and paediatrics.18
Considering drug pharmacokinetic, few data are available for patients younger than 18 years old, with limited information and guidelines.1,11,12 However, as reported in adults, suboptimal drug plasma concentrations have been reported.11,19 For children, the known PSC plasma concentration threshold values reported for adult patients (700 ng/ml for prophylaxis and 1000 ng/ml for therapy of IFIs) can be used. Furthermore, sub-optimal exposure could be effective because the drug accumulates in macrophages. Evaluating the role of protonic pump inhibitors, available information are contradictory.19
In the present work, we analysed PSC pharmacokinetics in children with IFIs receiving oral therapy, also considering separately the group of adolescent (age ≥13 years old). We showed that PSC levels had a high interindividual variability in both the evaluated groups. Moreover, our results reported PSC plasma median values lower than already reported in literature.13
We observed an inverse and statistically significant correlation among PSC Ctrough and drug dose in all the 26 enrolled patients (p=0.010; Figure 1) and in adolescent (p=0.003; Figure 2). There is a substantial variability in the dose required to obtain an adequate drug concentration in children as a result of the unpredictable PSC bioavailability.12 Döring et al. reported significantly higher levels with three times daily dosing compared with twice daily;20 moreover has been observed that higher dose do not result in proportional raising of drug exposure. In fact, attaining PSC target serum concentrations is limited by its saturable absorption which could lead to a difficult in increasing the serum concentrations. A study in adults based on the increasing PSC single doses reported a proportional increase in drug exposure with dose escalations between 50 and 800 mg, however, it results similar or reduced with doses of 1200 mg.21
Participants BMI, ethnicity, sex or age did not significantly affect PSC pharmacokinetics. On the contrary, a relationship between weight and a larger PSC volume of distribution was observed in a PSC population pharmacokinetic analysis.22 Moreover, it is known that gender-related differences, such as body size and muscle mass, may result in drug pharmacokinetic differences.17,23
Evaluating adolescent, we reported, for the first time, an inverse correlation between age and PSC plasma concentrations (p=0.015; Figure 3), as previously reported by Kohl and colleagues in a study on prophylactic PSC use in adult patients undergoing allogeneic hematopoietic stem cell transplantation.24 In addition, we observed the influence of sex on PSC levels (p=0.012; Figure 4). The pharmacokinetic of PSC varied between men and women and this could be the result of gender-based variations in CYP-mediated metabolism, the impact of sexual hormones on medication absorption, and variations in body fat percentage and age.23,25-27
Potential limitations to our study include its retrospective nature and a relatively small sample size; moreover, it lacks of a PSC dosing standardized protocol. Hence, further works applied to larger cohorts, are required to confirm the reported data.
To date, safety, efficacy and appropriate dosage of PSC in paediatrics have not been systematically investigated.4 This study reports the usefulness of TDM in children with IFIs and its significant clinical implications to achieve target serum concentrations, particularly after the first week of treatment, when steady-state is achieved.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
None.
Authors declare that there is no conflict of interest.

©2022 Allegra, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.