eISSN: 2576-4470


Review Article Volume 3 Issue 2
1Department of Biological Anthropology, Aix Marseille Univ, France
2Department of Biological Anthropology, Aix Marseille Univ, France
Correspondence: Philippe Stefanini, Department of Biological Anthropology, Aix Marseille Univ, France
Received: November 28, 2018 | Published: April 1, 2019
Citation: Stefanini P, Gaillard S. Social food to protect by putting in the common good this genomic sequence. Sociol Int J. 2019;3(2):225-227. DOI: 10.15406/sij.2019.03.00179
paracas, arthrospira, platensis, delivery, technology, platform, sequencing, scientific, track
The genome of spirulina strain "paracas" of South America better known scientists under the name species Arthrospira platensis has been sequenced by Sandrine GAILLARD Bio Tech Services technology platform service delivery, and registered on November 25, 2018 in the GenBank global genebank. Today, most producers and industrial spirulina think only use arthrospira platensis but this article demonstrates that indeed almost all known strains are arthrospira platensis except the "paracas" strain of South America. Apparently, the insight of the brilliant global cyanobacteria expert Dr. Ripley FOX was good. From all over the planet whether on the industrial or artisanal level as in France farmers mostly produce in brackish water this greenish food bacterium called "spirulina". It has many qualities: nutritional,1 environmental, agronomic, therapeutic. At the moment, having made a state of the art international no researcher knows interested in genome sequencing of the strain "paracas". That's why we started doing this sequencing with BioTechService. By moving towards this new scientific track, we wanted to better understand some professional questions and show that this new Spirulina should in the future be taken into account differently as well on the physiological, biological as on the productive level. This publication aims to be put in the common good to prevent the establishment of genetically modified organisms. By registering the genome at GenBank, we have chosen to make it accessible to everyone. This collaboration is also motivated by the desire to prevent the patenting of the genome of this spirulina from South America and will allow anyone interested to go faster and further in the many potential uses. These include nutritional applications, industrial ecology by carbon sequestration, energy production, composting or the production of molecules for therapeutic use.
Spirulina has long been considered a blue-green alga (Cyanophyceae) by botanists, but it is actually a cyanobacterium validated by microbiologists including Woese in 1987. It develops rapidly by photosynthesis in a brackish and warm aquatic environment of shallow depth. "From a systematic point of view, these are prokaryotes placed in the reign of Eubacteria".2 These cyanobacteria "were the incredibly active agents of the metamorphoses then undergone the terrestrial environment. They made it habitable, releasing little by little the oxygen which was to form the atmosphere, in other words the breathable air. As a result, they have allowed all plants, all animals, the man himself to make their appearance in this world ". There are many studies on its nutritional qualities concerning its impressive protein content (more than 50% of its dry weight), rare essential lipids, numerous minerals (world record for its exceptional quantity of iron available) and vitamins (all except ascorbic acid), as well as its growth rate in totally mineral environments, which attracted the attention of farmers in particular. Cellulose-free, spirulina is perfectly digestible, raw or dried. Numerous nutritional tests have proven the bioavailability of its micronutrients.1 m on the biological and agronomic level; its "sustainable" aspect is still highlighted, in particular because of its low water consumption and its high carbon dioxide absorption.
Spirulina also has therapeutic properties. It contains several molecules that have been studied for their biological activities. The immunostimulant and antiviral properties of Spirulina are particularly important in malnutrition. Note however those clinical studies on humans exist but are still too few.3 Some effects of spirulina have been mainly observed in the laboratory, or even in animals. It was first described by Wittrock and Nordsted in 1844 as Spirulina Jenneri platensis Nordsted. It is like tiny filaments (0.1 mm long) wound in turns. Appeared about 3.5 billion years ago, it is one of the oldest inhabitants of our planet and has been consumed for centuries by certain populations. This primitive creature, living fossil alive still lives in some salt lakes. Nowadays, spirulina is added to the usual diet. Added in the morning to orange juice, it also sprinkles on salad, yogurt, jam, dressing sauce. Anything is possible, as long as it is not cooked, which would reduce its nutritional efficiency. As well as being sincere, the smell and color of some dried spirulines are not very appetizing. However, fresh spirulina or capsule has almost no aroma and no taste. The man controls his production for only 40 years. Today in the West a whole commercial and production chain is in place to offer spirulina to consumers and in southern countries spirulina is offered to the malnourished by non-governmental organizations with very artisanal farms.
Genetics sequencing of the strain "Paracas" by comparison
Biological material
All strains analyzed come from the Paul Ricard Oceanographic Institute, Embiez Island, 83140 Six Fours les Plages, Var, France. The 2010 campaign consisted of 10 culture samples from 5 different spirulina strains: Ethia, Paracas, Lonar, Toliara, Laay; either 2 biological replicates per strain. The 2011 campaign consisted of 6 dried culture samples from 3 different spirulina strains: Paracas, Paracas XXL and Simbi; either 2 biological replicates per strain.
The different samples were stored at -80°C until their treatment.
Extraction of nucleic acids
For the 2010 campaign, one milliliter of each culture is taken and centrifuged for 2 minutes at 5000 rpm. For the 2011 campaign, 100 mg of dry culture is resuspended in 50 ml of ultrapure water, then 1 ml is taken and centrifuged for 2 minutes at 5000 rpm. The pellets of both campaigns are recovered and the extractions are carried out using the kit D Neasy Blood & Tissue kit (Qiagen) according to the instructions of the manufacturer. The nucleic acids (DNA) are eluted in 100 .mu.l of elution buffer. The quantity and quality of the DNAs are evaluated by spectrophotometry (quantity in ng /μl, quality according to the value of the Do260/Do280 ratio).
Amplification and sequencing of genetic markers
The genetic marker used is the amplification of the ITS region located between the genes coding for 16S rRNA and 23S rRNA. This polymorphic region makes it possible, after analysis of the sequences obtained, to classify the strains in different clusters: Cluster I or II and in subclusters: IA or B, II A or B. The protocol used is taken from the publication of Baurain.4 The reaction mixture is as follows for a final volume of 50 μl: 5 μl of 10X buffer (5PRIME); 1μl of DNTPmix (10mM, Invitrogen); 0.3μl Hot Master Taq (5U / μl; 5PRIME); 2.5 μl of primer 16S3'F (5'-TGY GGC TGG ATC ACC TCC TT) and 2.5 μl of 23S3'R long primer (5'-TCT GTG TGC CTA GGT ATC CAC CGT T) (10 μM; Eurogentec ); 25ng of DNA and qs of ultra pure water. The amplification program is as follows: a denaturation cycle at 94°C. for 3 min and then for 9 cycles: denaturation at 94°C. for 45 sec, hybridization of the primers at 57°C. for 45 sec, extension at 68°C. C for 2 min, then for 24 cycles: denaturation at 90°C for 45 sec, hybridization of the primers at 57°C for 45 sec, elongation at 68°C for 2 min. The amplification products are visualized using a UV trans illuminator after 1.5% agarose gel electrophoresis (TBE buffer, 150 V) and ethidium bromide staining. Then they are purified using the QIA quick PCR purification kit (Qiagen) following the manufacturer's recommendations. Each purified product is sequenced in both directions from the primers used for PCR by Genewiz.
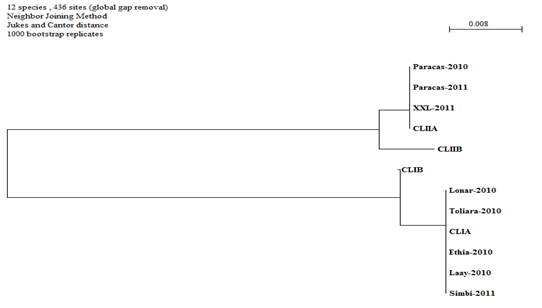
The consensus of the sequences obtained for each sample is obtained using Multalin software.5 The software Clustalw6 and Sea View/Phylo_win7 are used to obtain, from the alignment of the sequences, a dendogram according to the method of Neighbor Joining (1000 bootstrap).8–10
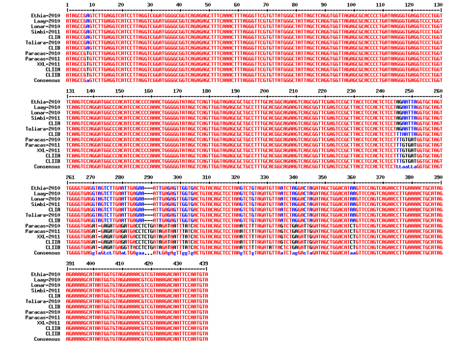
All the samples made it possible to obtain nucleic acids in concentrations ranging from 5 to 57 ng/μl for ratios (OD 260/Do 280) ranging from 1.6 to 1.9. The lowest concentration and ratio values were obtained with the Paracas samples for both sampling campaigns.11 The amplifiats of the ITS region correspond to the expected size of 550 bp. The alignment of the sequences obtained and of the characteristic sequences of the different clusters and subclusters makes 439 bp and makes it possible to differentiate the two clusters I and II and the subclusters IA, IB, IIA and IIB (Figure 1). There are 38 polymorphic sites in total, of which 2 sites make it possible to differentiate the IA and IB subclusters and 4 sites making it possible to differentiate between the IIA and IIB subclusters. These sites are the same as those found by Baurain.4 Figure 2 Dendogram constructed with the Neighbor-joining method,12–15 applied to a matrix of distance with a correction of Jukes and Cantor, from the ITS sequences of the 8 culture samples and the 4 sequences resulting from the data published by Baurain.4,16–20 This sequencing of the genome of spirulina strain "paracas" announces that it is the second spirulina and especially the first strain of South America to have been sequenced. The DNA sequence was analyzed from strains from CFPPA Hyères; it took not millions of reads piece by piece to reconstruct this genome.21,22
Having this genome in detail is a fundamental step in understanding how this atypical cyanobacterium works, said Philippe STEFANINI who followed Ripley FOX's intuition.23–25 The meeting with the brilliant geneticist Sandrine GAILLARD was fundamental to demonstrate these empirical observations. And indeed, this publication should certainly inspire dozens of researchers and new discoveries followed by publications in scientific journals.26 These genes give us clues for understanding the physiology and evolution of this spirulina but also for other cyanobacteria. It is by exploiting the sequencing data that a team of researchers will be able to determine, for example, that this spirulina has left the arthrospira platensis line earlier.27 Another interesting novelty for the phylogeny of spirulina is that sequencing has made it possible to identify new markers of genomic DNA (called SNPs for single nucleotide polymorphism).28,29 The consortium will be able to compare the contents of the genome with that of arthrospira platensis whose genome was sequenced in 2010, Biorigin SA and Fasteris,30 as well as a Hepia Specialized High School (HES-so//Geneva) represented by the research group. Plants and pathogens.
None.
The author declares that there are no conflicts of interest.

©2019 Stefanini, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.