Advances in
eISSN: 2378-3168


Research Article Volume 8 Issue 3
Faculty of Health and Human Sciences, Queen Margaret University, UK
Correspondence: Elena Bega, Faculty of Health and Human Sciences, Queen Margaret University, Edinburgh EH21 6UU, UK
Received: April 14, 2018 | Published: May 16, 2018
Citation: Bega E. Association between dietary calcium and dietary Vitamin D deficiency, body composition and bone mass in healthy adults. Adv Obes Weight Manag Control. 2018;8(3):168-173. DOI: 10.15406/aowmc.2018.08.00236
accrual
Keywords: dietary vitamin D, dietary calcium, body composition, bone mass
Bone mass is a composite measure, which includes contributions from bone size and its volumetric mineral density and also, is an established determinant of bone strength. In addition, the bone mass of a person in his later life, depends on the peak attained during skeletal growth and the subsequent rate of bone loss. This is evidenced by, mathematical models and through various long-term studies that have attended bone mass from childhood through adolescence, and have suggested that modifying peak bone mass will have biologically relevant effects on skeletal fragility in old age, this is also apparent in Figure 1.3
According to Cooper3 and Ilich4 bone mass is influenced by the diet, body composition, physical activity, age, weight and hormones. Some epidemiological data have shown that body mass index (BMI) is associated with high bone mass and also, a reduced risk of fractures. There are many explanations for this relationship, such as, body weight is thought to affect the bone mass by mechanical loading of the skeleton and by increasing the stress through muscle pull. Furthermore, as the relationship of body weight with bone mass is well known, some studies have shown that the relationship between the individual components of body weight (fat and lean tissue) and bone mass are equivocal.5 In addition, there are several studies which have indicated a positive association between lean tissue and bone mass,6 they have also, demonstrated, that both fat mass and lean mass contribute equally to bone mass especially in women.7 In addition, obesity can be associated with secondary hyperparathyroidism and with vitamin D deficiency due to inadequate Vitamin D3 from cutaneous and dietary sources because of its deposition in body fat compartments.6 By the age of 18 years old almost 90% of the skeletal mass is accumulated, this proves that the majority of bone mass is attained during young adulthood and adolescence. There are many factors that influence bone mass accrual during growth, some of those factors are genetic like the gender, the race, the endocrine, there are also, mechanical factors and some pharmacological agents and finally, dietary factors, especially calcium and vitamin D intake.8
According to Moyer9 there is no evidence, which confirms whether calcium and/or vitamin D supplementation causes greater benefit or harm in men and in premenopausal women. In addition, in postmenopausal women it is not recommended a lower dose supplementation (lower than 400 IU of vitamin D and 1gr of calcium/day) as there seems to be no difference in the risk of fracture. Finally, it is still unknown what can overdose/ higher dose cause. According to DIPART10 supplementations, only with vitamin D cannot prevent from fractures, but it is necessary to combine with calcium so that might. But in that situation there is a high risk of developing myocardial infarctions2 and also, stones in the kidneys.9
Vitamin D
Vitamin D is very important for calcium metabolism8 and it is also, associated with skeletal growth and resistance to bone, this correlation is demonstrated by rickets, which is a disease that appears in childhood and is characterized by abnormal development of the bone, and can be prevented by the fat-soluble factor D of the diet or by the exposure of the body to ultraviolet radiation.11 There are some epidemiological studies, which have demonstrated the importance of vitamin D for bone health, for neuronal system, immune functions8 and for prevention of certain cancers and cardiovascular diseases and hypertention.1 Furthermore, vitamin D deficiency has also been associated with autoimmune diseases such as multiple sclerosis and with diabetes mellitus type I.8
Calcitriol
Vitamin D status is indicated by the serum concentration of the hydroxylated form, of 25-hydroxyvitamin D (25-OHD). 25-OHD is converted to 1, 25-dihydroxy vitamin D8 or calcitriol11 in the kidney but it is also converted as an autorine/paracrine mode in many other cell types. More detailed, the principal action of calcitriol as a hormone in the human body is to act together with parathyroid hormone (PTH) for regulating the homeostasis of calcium concentration in blood. The low concentration of calcium in the blood or else hypocalcemia stimulates the secretion of PTH from parathyroid glands. PTH in turn, stimulates the 1-hydroxylase in the kidney and thus the 25-D3-ouch converts to calcitriol. Calcitriol then acts alone or together with the PTH, in the tissues, causing increased calcium and phosphorus concentration. The three main tissues- target of calcitriol are intestine, kidneys and bones, as shown in Figure 2.11
Dietary sources of vitamin D
Vitamin D is found mainly from foods of animal origin. More specifically vitamin D is provided by the liver (0,5-4,0μg/100g), the veal, beef and from eggs. In addition, other good sources of vitamin D are dairy products, such as, cheese (<1,0μg/100g), butter (0,3–2,0μg/100g) and milk (<1,0μg/100g), also, some kind of fish (5,0-40,0 μg/100g), such as, tuna, sardines, salmon and herring. There are also, foods which are enriched with vitamin D e.g. milk (0,8–1,3μg/100g) and margarine (8,0-10,0μg/100g). (GROPPER et al, 2008)
Recommendations for vitamin D
Vitamin D is known as a sunshine vitamin, because is produced in the skin with the help of the ultraviolet B (UVB) energy.12 According to the European Food Information Council13 the recommended dietary allowance for vitamin D is 5μg or 200IU/ day. The recommended daily amount for teens and adults, ages 19 to 7015 years old are15µg or 600IU)14 and also the same units for women during pregnancy or breastfeeding. For elderly older than 71 years old the recommendations are 20μg or 800IU/day, as well as in postmenopausal women. Recommendations are changing in patient with very low vitamin D status (such as, people14 who may not be able to access the sun exposure etc.), the dose should be increased to 4,000IU/day. According to the UK National Health Service, infant and children aged six months to five years old, pregnant or lactating women, and also, elderly people who deprived the sun, it is necessary to take daily vitamin supplements in order to ensure sufficient vitamin D intake. The general population receives enough vitamin D by following a diet which is based on the recommended daily amount for dietary vitamin D and from sunlight.16 Furthermore, is required about 10 minutes of exposure to the summer sun in the face and hands, in order to produce 10μg or 400IU (but, it is not able for all population groups).11
Vitamin D deficiency
Vitamin D deficiencies are common in people who deprived the sunlight, in patient with intestinal disorders and with patient with liver or kidney diseases that reduce the conversion of vitamin D to its active form, (1,25(OH)2D).17
The clinical features of vitamin D deficiency are muscle weakness, bone deformity, hypotonia, fracture and bone pain and they are depending on the age of onset.18 According to Pekkinen8 some epidemiological studies over the last decade showed that, vitamin D deficiency is common in children and in adolescents, in these young age groups, complications of vitamin D deficiency can appear with significant delay and it can also remain unnoticed.8 Furthermore, it is well known that persistent severe vitamin D deficiency is associated with the bone abnormalities of rickets and osteomalacia.19 Rickets occurs in infants and children and is characterized by the lack of bone plating, while, osteomalacia occurs in adults and is caused by a deficiency of vitamin D, which leads to reduced plating.11 The main role of vitamin was considered to be the calcium absorption, but lately, researchers found many other roles of vitamin D.
Calcium
Calcium is the most abundant divalent cation in the human body and it constitutes approximately 1.5% to 2% of the total body weight or 1400gr calcium content in the body.11 As you can see in Figure 3 calcium is the fifth most abundant element in the human body.20 About 99% of the calcium is found in the bones and teeth, while the remaining 1% distributed in the intracellular and extracellular fluids.11
Dietary sources of calcium
The best dietary sources of calcium are milk (1 portion is 306mg/1 cup) and dairy products, mainly yogurt (452mg/8oz) and cheese (452mg/1,5oz), vintage seafood, such as salmon (181mg/3oz), mussels, oysters and sardines, some green leafy vegetables, such as broccoli, cabbage, cauliflower, iceberg lettuce (97mg/1 head) and turnip. Finally, good calcium sources are legumes and fruits (e.g. beans 154mg/1 cup and oranges 72 mg/1 cup).11,17, 21 Other foods that are good sources of calcium are those which have been enriched with calcium, such as juice (orange juice) or bread.
Recommendations and deficiency symptoms of calcium
According to Gropper11 the daily recommendation of calcium for adult men and women, ages 19-50years old, are 1000mg/day, and for adults over the age of 50 years old, the recommendations are 1200mg/day. While, calcium intakes greater than 1500mg/day cause no further increases in retention of calcium, instead, it is simply excreted (Figure 4).
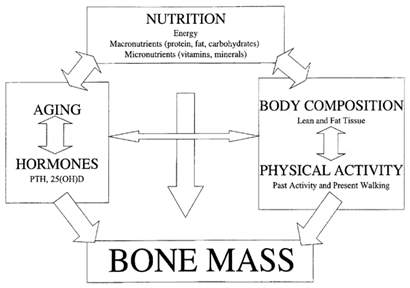
Figure 4 shows the path diagram, with the possible interaction among bone mass and various lifestyle and biological variables. Also, the arrows indicate the direction of the interaction.29
Inadequate intake, malabsorption, or excessive loss of calcium can cause rickets in children. In addition, low calcium levels in blood can cause tetany. Tetany manifested by intermittent muscle contractions, especially in the leg and hands muscles. Some often signs of tetany are muscular spasms, muscle pain and delusions such as the numbness tingling in the hands and feet.
Furthermore, inadequate calcium intake can cause osteoporosis in adults and inadequate calcium intake for a long period of time is associated with the development of hypertension, colon cancer and obesity or weight gain.11,22 Osteoporosis is defined by the World Health Organization (WHO) as ‘‘a bone mineral density (BMD) of 2.5 standard deviations or more below the mean peak bone mass (average of young, healthy adults). The most widely validated technique to measure BMD is dual energy X–ray absorptiometry (DXA), and diagnostic criteria based on the T-score for BMD are a recommended entry criterion for the development of pharmaceutical interventions in osteoporosis.’’ Table 3 shows the diagnostic criteria for osteoporosis.23
Body composition and bone mass
A number of studies from India have shown that body weight is one of the most important determinants of bone density and may be protective against fractures. According to Reddy’s et al.,5 research, high fat mass is associated with higher bone mineral density but there was a negative relationship with vitamin D serum in women. These findings may indicate that mechanical loading effect of fat mass, lean mass and body weight are more important for bone mineral density than low serum level of vitamin D. So, finally, fat mass is very important for bone mineral density especially in women. All the above, suggest that more information is needed for the effect of dietary Calcium and vitamin D intake on Bone Mass, especially among young adults that are currently on the peak of their Bone Mass.
Aims and objectives
The main objective of this research is to examine the association of dietary calcium intake and dietary vitamin D intake with BMI and bone mass in healthy adults (over the age of 18 years old). The expected outcome is that higher dietary calcium and dietary vitamin D intake correlate with increased bone mass. The dietary parameters were assessed though validated tools (Food Frequency Questionnaires) and 3-days food diaries. Bone Mass and Fat Percentage were assessed through Bioelectrical Impendance.
Study design and subjects
The present study is a cross sectional study that took place in Athens, Greece. The study sample consisted of 17 healthy subjects, (%males), all adults. A first meeting with the participants took place before the measurements, in order to present them the aims of the research project and make them familiar with the procedure. Inclusion criteria consisted of subjects being able to live independently, non-smokers, free of chronic disease (kidney stones, hypertension, diabetes, severe osteoporosis and cancer) and not taking diuretics or medications known to affect bone metabolism, including but not limited to hormone replacement therapy, bisphosphanates, corticosteroids, insulin or anticonvulsants for the past 3 months. Finally, all the participants must sign a consent form. All subjects that did not meet these criteria were excluded from the study sample. Measurements took place within one morning.
Anthropometry (weight, height, BMI)
The weight of subjects was measured in light, indoor clothing and without shoes and height was measured in cm, with stadiometer, putting people without their footwear in standing up position, to get correct results.24 After collecting height and weight from the individual, the Body Mass Index (BMI kg/m2) was calculated to define obesity status. BMI can be calculated according to the WHO’s classification system: Metric BMI(kg/m2)=weight in kg/(height in m)2. The categories of the BMI are apparent on Table 4.25
Body composition and bone mass measurements
Bone mineral mass (BMM) and % of body fat will be measured with Bioelectrical Impedance Analysis (BIA).26 BIA is one of the most accessible and reliable methods for estimating the percentage of fat and fluids in the human body. The resistance or impedance to the signal is measured by BIA as it goes through the water that is in fat and muscle of the body. The amount of water in a person’s body is proportional to his size of muscle. It is easier for the current to pass through a person’s body if there is much water in it, but if the amount of fat is greater then, the current will have more resistance to pass through. Finally, BIA doesn’t hurt and it is absolutely safe, as the signals which are used in the body fat monitors cannot be felt, neither by a child nor by an adult.26 Tanita is a new, easy, fast and less intrusive way of measuring BIA, it also, includes a precision scale which makes this process very simple. First of all, the person should enter his height, age and gender and then should step onto the platform. Thereinafter, there are electrodes adhered in the person’s foot sensor pads, which send a low and safe signal through the body. Also, weight and body fat content is calculated automatically in less than a minute. Finally, all UltimateScales and Body Fat Monitor/Scales feature Tanita's patented BIA method.26
Dietary assessment
Except from anthropometry and Bioelectrical impedance analysis, the participants filled a food frequency questionnaire (FFQ) that is used to assess dietary calcium intake.27 Also, participants kept a 3 day food and drink diary. The calcium as well as the vitamin D daily intake was assessed with imputing their diaries into Diet Analysis software that provides estimation of daily micronutrients’ intake.28 The participants were instructed individually how to complete the records and to choose a typical day for reporting.29
Normality was examined graphically through histograms. Continuous variables were not normally distributed and thus, are presented as medians (interquartile range). Comparisons between males and females were performed using non-parametric Mann-Whitney test for sum of ranks. Correlations between continuous variables were performed using Spearmann’s rho, due to not normal distribution. Statistical analysis was further stratified into males and females. Subjects with missing values were excluded from the analysis. All statistical analyses will be performed using SPSS (data analysis software system), version 17.0.
The study sample consisted of 17 subjects (71%/12 females) with a mean age of 22±2 years. The measured characteristics (mean values, minimum and maximum and also p value) of the subjects are presented in Table 1, in respect to participants’ gender. As it is presented in Table 1, age, BMI, daily vitamin D intake, daily calcium intake, daily energy intake and fat percentage had no difference between males and females (as p-value>0.05). Males had significantly higher waist circumference than women (90cm vs.76cm respectively, p<0.001), as well as higher Bone Mass (3.5kg vs. 2.2kg respectively, p=0.001).In a further step of the analysis, the correlations between bone mass and age, BMI, Waist circumference, daily vitamin D intake, daily calcium intake, daily energy intake and fat percentage are presented in Table 2.
As it can be seen in Table 2, increased BMI was positively associated with Body Mass (rho=0.639, p=0.006), waist circumference (rho=0.778, p=0.001) and energy intake (rho=0.535,p=0.031). No other significant correlations were revealed with Bone Mass (all p=values>0.05).
Due to important gender differences as regards to the Bone Mass (shown in Table 1, p<0.001), the aforementioned analysis was further stratified by gender, as presented in Table 3. As it can be seen in Table 3, no significant correlations were revealed in contrast with Table 1, between the daily calcium intake and the age of the participants, their BMI, their daily vitamin D intake and their fat percentage. The same pattern was present for both sexes (all p-values>0.05).
|
Males (n=5) |
|
Females (n=12) |
|
p |
||||
|---|---|---|---|---|---|---|---|---|---|
|
Mean values |
(min,max) |
|
Mean values |
(min,max) |
|
|
||
Age (years) |
22 |
(22,24) |
|
22 |
(21,24) |
|
0.383 |
||
Body Mass Index (kg/m2) |
25 |
(23,30) |
|
21 |
(19,24) |
|
0.104 |
||
Waist circumference (cm) |
90 |
(86,105) |
|
76 |
(69,82) |
|
0.001 |
||
Bone Mass (kg) |
3.5 |
(3.2,3.7) |
|
2.2 |
(2.1,2.3) |
|
<0.001 |
||
Vitamin D (mcg) |
0.31 |
(0.0,0.69) |
|
0.03 |
(0.01,0.32) |
|
0.799 |
||
Calcium (mg) |
1475 |
(830,3166) |
|
935 |
(763,1090) |
|
0.234 |
||
Energy intake (kcal) |
1684 |
(1389,2282) |
|
1468 |
(1090,1678) |
|
0.160 |
||
Fat percentage (%) |
19 |
(13,29) |
|
20 |
(19,35) |
|
0.328 |
||
Table 1 Measured characteristics of the participants in respect to their age
*p-values derived from Mann-Whitney comparisons
Characteristic |
Spearmann’s rho, p |
Age (years) |
0.127,0.626 |
Body Mass Index (kg/m2) |
0.639,0.006 |
Waist circumference |
0.778,0.001 |
Vitamin D (mcg) |
-0.006,0.981 |
Calcium (mg) |
-0.006,0.981 |
Energy intake (kcal) |
0.525,0.031 |
Fat percentage (%) |
0.035,0.894 |
Table 2 Correlation between Bone Mass and the measured characteristics of the participants
*p-values derived from Spearmann’s rho correlation coefficients
|
Males (n=5) |
Females (n=12) |
Age (years) |
-0.707,0.182 |
0.290, 0.361 |
Body Mass Index |
-0.700,0.188 |
-0.245, 0.443 |
Vitamin D (mcg) |
-0.410,0.393 |
0.021, 0.948 |
Bone mass (kg) |
-0.718,0.172 |
-0.422,0.172 |
Fat Percentage |
-0.700,0.188 |
-0.154, 0.632 |
Table 3 Correlation between daily and dietary calcium intake and anthropometric characteristics in respect to subjects’ gender
*p-values derived from Spearmann’s rho correlation coefficients
Weight Classification |
|
|
BMI (kg/m2) |
Underweight |
|
|
<18.5 |
Normal |
|
|
18.5–24.9 |
Overweight |
|
|
25–29.9 |
Obese: Class 1 |
|
|
30–34.9 |
Obese: Class 2 |
|
|
35–39.9 |
Obese: Class 3 |
|
|
≥40 |
Table 4 shows the categories of BMI25
The present study revealed that increased BMI was positively associated with Bone Mass, but this finding lost significance when we stratified into males and females. Males had significantly higher waist circumference than women (90cm vs.76cm respectively, p<0.001), as well as higher Bone Mass (3.5kg vs. 2.2kg respectively, p=0.001) the same results had and Nieves’s research in 2004. This could be attributed to the fact that males have higher Bone Mass than females due to different hormonal cycles and actions.30 Thus, male gender is the most important predictor of Bone Mass, as the only characteristics that differed between males and females were Bone Mass and waist circumference, both attributed to gender differences. Also, increased Body Mass Index was positively associated with Body Mass (rho=0.639, p=0.006), waist circumference (rho=0.778, p=0.001) and energy intake (rho=0.535,p=0.031)As regards to the role of dietary calcium intake, no important association was revealed with Bone Mass, neither for the total sample, nor for the males and females alone. Moreover, dietary vitamin D intake was not associated with Bone Mass, in both genders.
As regards to the role of BMI on Bone Mass, it has been widely accepted that increased BMI has been associated with increased Bone Mass and increased Bone density. This is mainly attributed to the increase load of extra weight on bones, which leads to increased bone genesis and reduced bone decline.31 This was confirmed in the presented analysis, but lost significance when the analysis was further split into genders. This could have occurred either due to reduced power when splitting the sample, or due to the confounding rile of gender.
Daily vitamin D and daily calcium intake were not proved to have significant association with Bone Mass, which is in accordance with other studies, but in discordance with studies that focus on women. It could be summarized that for subjects of younger ages, the Bone Mass is not associated with micronutrients’ intake and that men that have almost the same hormonal profile through lifestyle, but women are a different issue. Findings from studies in women have associated dietary and supplementary intake of both calcium and vitamin D with decreased risk for osteoporosis and Bone Mass decline.1 The presented study performed the analysis for subjects with a mean age of 22 years, which probably lead to the null findings.
The presented study apart from its strengths bears several limitations. The study sample is small, which cannot lead to safe estimations and conclusions, but, the majority of studies that include measurements such as bioelectrical impendance do not have much more participants, so this study is comparable to other relevant studies. Moreover, the cross-sectional character of the presented study is not safe for etiological assumptions. Although, the Bone Mass is a parameter that does not easily change, so the measurement can be treated as unchanged for the latest years. As regards to the vitamin D intake, it is very difficult to take into account the non-dietary intake, but the purpose of this study was to investigate the dietary intake, so, 3-day diary was used to assess the vitamin intake and software that can estimate the specific intake. Dietary intake for both vitamin D and calcium cannot be fairly suggested by 3-days diary, because there is a high individual variance of food-intake within the year, but, this is the most used approach for nutrition epidemiology, so the results are comparable to other studies. Despite the aforementioned limitations, the present study used validated tools and standard procedures that have been already presented and discussed, in order to achieve the smallest possible bias.32
In conclusion, in this small study group (N=17) there appears to be no correlation between calcium and vitamin D intake for skeletal structure and body composition parameters of young adults. More prospective studies or even clinical trial that will focus on the increase of specific nutrients’ intake could provide more specific details on this issue.
The authors declare there is no conflict of interest.

©2018 Bega. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.