Advances in
eISSN: 2378-3168


Research Article Volume 13 Issue 4
College of Medicine and Graduate Studies, University of Science Arts and Technology, Montserrat, BWI
Correspondence: Orien L Tulp, College of Medicine and Graduate Studies, University of Science Arts and Technology, Montserrat, BWI
Received: August 23, 2023 | Published: September 8, 2023
Citation: Tulp OL. Differential depot-specific parameters of adipose tissue cellularity and thermogenesis in preobese congenic LA/Ntul//-cp rats. Adv Obes Weight Manag Control. 2023;13(4):81-85. DOI: 10.15406/aowmc.2023.13.00398
To determine the effects of adipose tissue development during early normal postweaning growth, groups of lean and obese LA/N//tul-cp rats were subjected to measures of caloric intake, linear growth and of adipose tissue mass, cell lipid content, and adipocyte number in principle adipose tissue depots at 6 weeks of age, prior to the expression of significant adiposity. Live body weights and daily caloric intake of obese > lean by 6 weeks of age, but parameters of linear growth were similar in both phenotypes. Resting metabolic rate (RMR) and the thermic response to norepinephrine (NE, 200 µg/KG BW, s.c.) of lean >> obese rats. Adipose tissue mass and combined fat pad mass:body weight ratio of Dorsal Subcutaneous (DOR), Retroperitoneal (RP), Gonadal (GON), and Interscapular brown adipose tissue (IBAT) depots were greater in the obese than lean phenotype, with the greatest magnitude of increase in the DOR subcutaneous depot. Adipocyte number was modestly greater in DOR subcutaneous depot, while adipocyte lipid content was greater in the RP and GON depots of obese but not lean rats. The IBAT mass and proportion of IBAT:body weight of obese >> lean. These results are consistent with the differential onset of adipose tissue depot growth initially occurs via hyperplasia in the dorsal subcutaneous depot and via hypertrophy in the RP and GON abdominal depots, while lean tissue development was of similar magnitude in both phenotypes at this age.
Keywords: obesity, adipocyte hyperplasia, hypertrophy, IBAT, rat
Obesity occurs in the congenic LA/Ntul//-cp rat as the result of the autosomal epigenetic expression of the corpulent trait for obesity (the -cp trait) in association with hyperphagia, a decreased capacity for nonshivering thermogenesis, and develops differentially in subcutaneous and abdominal fat pad depots by processes of hyperplasia and hypertrophy of adipocytes at 6 weeks of age, and before the onset of overt obesity has developed. Once formed, differentiated adipocytes can remain active as lipid storage depots virtually indefinitely, to accommodate the energy needs of the organism as needed during both energy privation and excess. In contrast, preobese indices of linear growth were similar in both phenotypes.
The processes of adipose tissue development have gained much overdue attention in recent years, as the incidence of overweight conditions has approached epidemic proportions throughout the globe, but particularly so in Westernized nations.1,2 In addition, the onset of excess body fat accretion now has an onset during adolescence or before, earlier in life than was prevalent in earlier generations.2 The process of excess body fat accretion occurs via hyperplasia and hypertrophy of preadipocytes, and likely develops at different rates in different adipose tissue depots.3,4 Although excess adiposity and fat distribution overall is commonly linked to potentially adverse health outcomes, different adipose depots appear to contribute to the adverse outcomes differentially, with abdominal depots exerting the greatest pathophysiologic impact.5–7 The subcutaneous depots are often referred to as upper body fat vs lower body fat, with the lower fat accretion being more resistant to weight loss, but of lesser apparent significance with respect to pathophysiologic contributions, while visceral abdominal depots have been associated with the greatest risk toward the development of adverse outcomes.5–7 It is now well established that excess body fat accretion occurs via processes of hyperplasia and cellular hypertrophy as preadipocytes take on added lipid, but the physiologic and endocrinologic signals that facilitate the two processes of fat accretion remain unclear, as are the depot specific mechanisms that ultimately facilitate adipose tissue expansion in the various anatomic sites, or the initial age of onset of lipid acquisition in the various anatomic locations.6–8 Thus, the purpose of the present study was to characterize the status of adipose tissue hyperplasia and hypertrophy during the initial stages of body fat accretion in a congenic animal model that is highly predisposed to obesity as the result of epigenetic expression of an obesity trait (the -cp trait) in the absence of other comorbidities common to the obese state.9–12 Once formed, differentiated adipocytes can presumably remain active lipid storage depots virtually indefinitely, to accommodate the energy needs of the organism as needed during conditions of both energy privation and energy excess. Moreover, adipose tissues are now known to exhibit endocrine actions which contribute to mechanisms of energy metabolism and energy balance, including caloric satiation. Familial predisposition i.e. genetic predisposition has also been shown to contribute to parameters of body composition including the propensity for development of obesity.8
The development of obesity in the congenic LA/Ntul//-cp rat strain occur via the autosomal transmission and epigenetic expression of an obesity trait (the -cp trait) and occurs in one quarter of the offspring of breeding populations that are heterogenous for the -cp trait by early adulthood, regardless of the diet that they may have consumed during their lifespan.3,9–13 This strain was developed from a cross between the Koletsky rat and a longevity prone strain of NIH Lister/Albany rats, followed by multiple cycles of backcrossing at the Small animal genetics laboratory of the NIH to attain congenic status, whereby the only surviving trait from the Koletsky donor strain was the demonstration of the -cp trait.10,11 The resulting animals demonstrate moderate glucose intolerance but remain euglycemic and non-hypertensive, in contrast to other strains that express an autosomal trait for obesity.3,12,13 Thus, the LA/Ntul//-cp stain is an appropriate animal model to investigate the characterization of adipose tissue depots in subcutaneous and visceral depots before the onset of obesity.
Groups of female lean and obese LA/Ntul//-cp rats (n= 6 rats/phenotype) were selected from the breeding colony at 6 weeks of age, housed in littermate pairs (1 lean+1 obese). Measures of resting and norepinephrine stimulated VO2 obtained in fasting animals (NE dose 200 µg/kg BW, s.c.) with a Collins small animal respirometer at thermal neutrality (30°C) as described previously.3,4,13 Rats were sacrificed by decapitation, and principle adipose tissue depots excised in their entirety. Tissues were weighed to the nearest mg, and representative weighed aliquots from each depot and subjected to measures of adipose tissue cellularity. Measures of caloric intake of Purina Rat Chow # 5012 corrected for spillage in individually housed animals were obtained over a 24-hour period in metabolic cages at 5.5 weeks of age; Adipose cell lipid content and cell number in osmium tetroxide fixed specimens via the method of Hirsch and Gallian as previously reported in our laboratory.13–16 Adipocyte lipid content, expressed as micrograms of lipid per adipocyte were determined gravimetrically via the procedures of Dole and Meinertz.17 Data were analyzed via descriptive statistics and comparisons were made by students unpaired ‘t’ test for unpaired comparisons and Pages L test for trend analysis where a significance level of < p = 0.05 could be established by the Students ‘t’ test.18,19 This study was approved by the Institutional Ethics, Animal Care and Use Committee.
The biometry of the lean and obese phenotype are depicted in Figure 1A, and indicate that initial body weights were similar at weaning but were modestly greater in the obese phenotype by 6 weeks of age. In contrast, linear indices including torso length and tibia length were similar in the two phenotypes. In addition, both digitorum longus muscle and cardiac weight were greater in the obese phenotype, proportionally consistent with the greater body weight bearing impact respectively of the obese phenotypes. Despite the presence of hyperphagia and increased IBAT mass in the obese phenotype, as depicted in Figure 1B, measures of resting and norepinephrine-stimulated thermogenesis were significantly decreased in the obese phenotype.
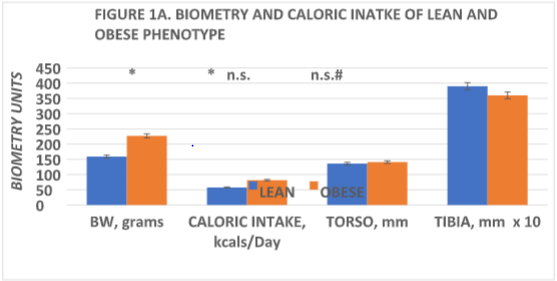
Figure 1A Data are mean ± 1 SEM, n= 6 rats/phenotype. * = p + < 0.05; n.s. = not significant at p = <0.05), comparisons determined via Students unpaired ‘t’ test; # = p = < 0.05 by Pages L Test for trend analysis; Grams live weight prior to sacrifice; Daily caloric intake at 5 and 1/2 weeks of age; Torso and Tibia measurement obtained to the nearest mm. with a micrometer after sacrifice. Body weights at weaning were 37±2 g in lean, 40±2 g for the obese phenotype (p = n.s.). Tibia measurements multiplied x 10 for ease of illustration.
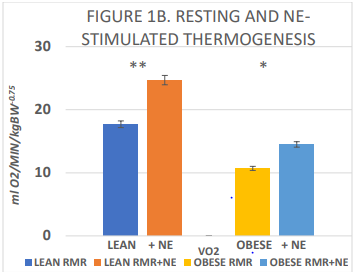
Figure 1B Resting and norepinephrine stimulated thermogenesis in lean and obese rats.
Data are mean ± 1 SEM, n=6 rats/group, expressed a ml O2/minute/kg BW -0.75 to compensate for differences in surface area between lean and obese phenotypes. * = p = < 0.05; ** = p = < 0.01. via Students unpaired ‘t’ test. Measures of VO2 obtained in fasted rats; NE stimulated VO2 following
administration of 200 µg of Norepinephrine (NE), s.c. in the mid upper dorsal region. The difference between lean and obese RMR = p < 0.05.
The adipose mass of the principal fat depots is depicted in Figure 2 and indicates that the absolute mas of the depots was greater in the obese than the lean phenotype in all depots measured, with the qualitatively greatest increase in the dorsal subcutaneous depot (~6x greater). In addition, the IBAT mass was also significantly 2-fold greater in the obese than the lean phenotype. The increase in fat accretion in the varipus depots is depicted in Figures 3 & 4, reflecting adipocyte lipid content and adipocyte number per depot respectively. In figure 3, adipocyte lipid content as an indicator of cellular hypertrophy was significantly greater in the abdominal retroperitoneal and gonadal depots of obese animals, and a trend toward greater cellular lipid content was noted for the dorsal subcutaneous depot in the obese phenotype. In contrast, adipocyte number per depot is depicted in Figure 4, and indicates that adipocyte number was significantly greater only in the dorsal subcutaneous depot, while adipocyte numbers in the retroperitoneal and gonadal depots remained similar in both depots, with only a modest trend toward greater number in the retroperitoneal depot. Thus, adipocyte hyperplasia was demonstrated to occur in the dorsal subcutaneous depot where the cell numbers were nearly twice as numerous as were observed in their lean littermates. When adipose tissue development is expressed as a function of body weight (Figure 5), thereby representing the combination of adipocyte hypertrophy, hyperplasia and supporting stromal tissues, the greatest increase averaging a 14-fold increase was noted in the subcutaneous depot, with lesser magnitude of increase (averaging ~9-fold increase) in the abdominal depots at this age. In contrast, the IBAT mass of obese rats was 2.3-fold greater in the obese than the lean phenotype, indicative of postweaning nutritional or thermogenic stimuli contributing to the IBAT tissue growth and cellular proliferation prior to full expression of obesity.
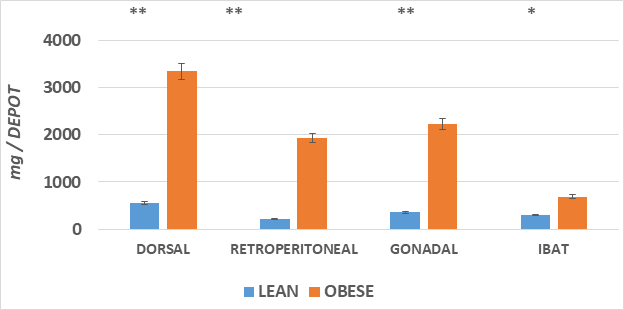
Figure 2 The adipose mass of the principal fat depots is depicted.
Data are mean ± 1 SEM, n = 5-6 rats/phenotype, expressed as milligrams per depot.
*comparison by students unpaired t test. * = < 0.05;** = < 0.01.
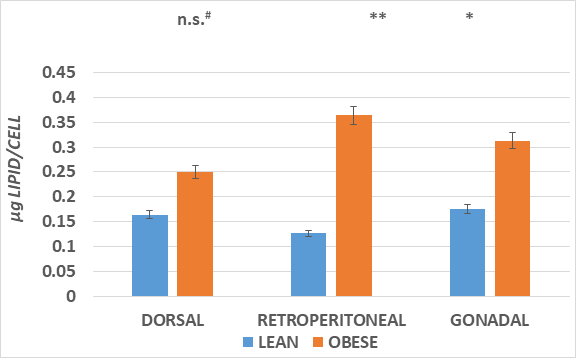
Figure 3 The increase in fat accretion in the varipus depots is depicted.
Data are mean ± 1 SEM, n = 5-6 rats/phenotype, expressed as micrograms of lipid per cell.
* Comparisons by students unpaired t test. * = < 0.05;** = < 0.01. n.s = not significant at < 0.05; #= p=<0.05 by Pages L test for trend analysis.
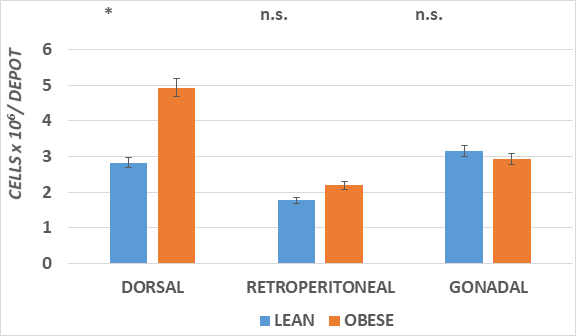
Figure 4 The increase in fat accretion in the various depots is depicted.
Data are mean ± 1 SEM, n = 5-6 rats/phenotype, expressed as cell number x 10-3 per depot.
* comparisons by students unpaired t test. * = < 0.05;** = < 0.01. n.s = not significant at < 0.05
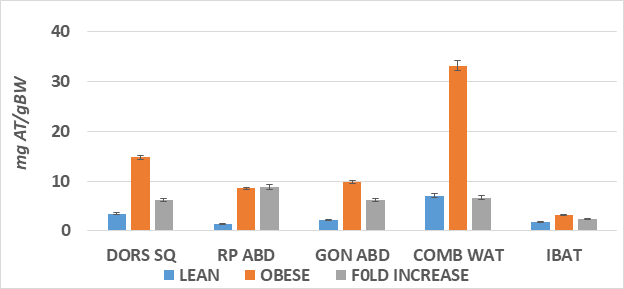
Figure 5 Ratio of adipose tissue to body weight in four depots.
Data are mean ± 1 SEM, n= 6 rats/group, expressed a milligrams adipose tissue / depots per gram of body weight at the time of sacrifice. DORS SQ, dorsal subcutaneous depot; RP ABD, retroperitoneal abdominal depots; GON ABD, gonadal/paraovarian abdominal depots; COMB WAT, sum of DORS SQ, RP ABD and GON ABD depots, BW; IBAT, Interscapular brown adipose tissue; Fold increase, magnitude of increase in obese compared to lean littermates.
The results of this study indicates that the onset of the process of adipocyte hyperplasia and hypertrophy commence early during postweaning growth in the non-diabetic LA/Ntul//-cp strain of rat. The increases in adipose tissue development in the white adipocyte tissue depot mass were accompanied by similar increases in the interscapular brown adipose tissue depot as well, although detailed measures of brown adipocyte cellularity were not included to confirm if the increase was secondary to hyperplasia, hypertrophy or some combination of the two processes. Adipose tissue has been shown to release a number of hormonally active peptides, including leptin and others,8 but the age of onset of those hormonally active peptides is unclear or if they may have been contributory to the onset of adipose tissue expansion in the obese phenotype of this strain. In previous studies, the obese phenotype was shown to demonstrate chronic, lifelong hyperphagia from an early age, with the earliest measurements at 6 weeks of age and which was further increased when offered a highly palatable cafeteria feeding regimen.4,18,19 Tulp et al. reported hyperplasia of IBAT depots following various feeding regimens including protein restricted but calorically adequate diets in normally lean Sprague Dawley rats, resulting in increases in IBAT cellularity and IBAT mass, with only modest changes in adipocyte lipid content.20 In the obese phenotype of this and other obesity-prone strains, hyperphagia and increases in IBAT mass and cellularity in the presence of an impaired thermogenic response to diet and exogenous norepinephine are a common observation, and may signal a physiologic attempt by the obesity-prone phenotype to increase their capacity for energy expenditure via non shivering thermogenesis, thus accounting for the greater IBAT mass observed in the present study.20–22 The primary physiologic function of brown adipose tissue is energy expenditure in response to factors of diet and environment, in contrast to depots of white adipose tissue that have a physiologic function of energy storage particularly during periods of excess energy intake or decreased energy expenditure or some combination of the two functions. The majority of adipocyte lipid accumulation is presumed to derive from hepatic or preformed lipids, in addition to de novo lipogenesis in adipose tissue.
Adipocytes, once formed, presumably may survive throughout most or all of an animal’s projected lifetime.3,13 Preadipocytes in some depots may continue to differentiate throughout much of an animal’s lifespan while in other depots including gonadal depots, continued adipocyte proliferation into later adulthood has not been demonstrated. Thus the gonadal adipocyte numbers in the present investigation were similar in lean and obese phenotypes, suggesting that increases in the gonadal and retroperitoneal depot mass cellularity were limited to increasing cellular lipid content at the age studied. In contrast to the dorsal subcutaneous depot where the increases in both cell lipid content and adipocyte number were present, with the hyperplasia of greater magnitude than the cellular lipid content. Thus, the factors that modulate increases in adipocyte hyperplasia and hypertrophy differ if subcutaneous vs abdominal depots during the early stages of lipid accretion and development of obesity.
Despite the differences in adipose tissue development observed, measures of net linear growth were similar in both phenotypes, albeit it with a tendency toward greater muscle mass likely as a result of the greater weight bearing potential, greater workload and expanded cardiovascular parameters in response to the greater cardiovascular workload associated with the obese state, also reflecting an adaptational shift in substrate oxidation in cardiac and other tissues toward greater utilization of fatty acids at least in part as a reflection of insulin resistance in peripheral tissues.23–26
The results of this study demonstrated differential responses regarding adipocyte hyperplasia and hypertrophy during the early, postweaning development of principal fat depots at the onset of epigenetic expression of the obese phenotype. The greater fat pad mass in the obese phenotype was positively correlated with the preobese hyperphagia and decreased resting thermogenesis in the obese phenotype. While all of the fat depots examined were significantly greater in the obese than the lean phenotype, the initial increase in mass in the Dosal Subcutaneous depots was predominantly by hyperplasia, while in the deeper abdominal gonadal and retroperitoneal depots, the initial increases were predominantly via hypertrophy. These observations may signal metabolic differences in lipid storage and accretion in the different lipid reserves of the developing lean and obese phenotypes, and which may impact on the progression of pathophysiologic sequela later in life. Longevity has been shown to be significantly longer in the lean than the obese phenotype in this strain, likely secondary to metabolic factors that become expressed in the obese phenotype. Measures of resting and norepinephrine stimulated thermogenesis were greater in the lean than the obese phenotype. Despite the significant differences in lipid accretion in the various adipose tissue depots studied, parameters of lean tissue growth including skeletal dimensions remained similar in both phenotypes, and are indicative of phenotype differences in the economy of resting, diet, and norepinephrine stimulated energy metabolism, favoring a greater facility for lipogenesis and fat accretion in the obese phenotype in advance of the frank expession and development of obesity.
The author thanks the University of Science Arts and Technology, Montserrat for the Institutional resources, Mr. Peisong H for animal husbandry, Dr. Dubin S for veterinary support, and Ms. Sandler C and the late Dr Michalis OE IV for technical support.
The author declares that there is no conflicts of interest.
None.

©2023 Tulp. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.