Advances in
eISSN: 2378-3168


Research Article Volume 9 Issue 3
Food Science and Technology Department, Zamorano University, Honduras
Correspondence: Adriana Di lorio, Food Science and Technology Department, Panamerican Agricultural School, Zamorano University, San Antonio de Oriente, Honduras, Tel 50494813975
Received: April 30, 2019 | Published: June 5, 2019
Citation: lorio AD, Hernández A, Ariza E, et al. Effect of the use of stabilizers on the performance and physical–chemical and sensory characteristics of Zamorano cream cheese. Adv Obes Weight Manag Control. 2019;9(3):79-89. DOI: 10.15406/aowmc.2019.09.00279
The purpose of the study was to assess the body composition of Honduran employees of two institutions. It was a cross-sectional descriptive study of prevalence, and the sampling was non-probability voluntary composed of 41 women and 29 men. There was a significant difference for glucose between the employees of the two institutions (p<0.05) and likewise for the systolic pressure between the employees of the two institutions. Hemoglobin was associated with higher levels of visceral fat (r=0.40232, p=0.033), muscle mass (r=0.4682, p <0.0001) and cellular quality (r=0.5396, p<0.0001). There was a positive relationship between cholesterol and people’s age (r=0.2643, p=0.0248) of institution 1 and 22% of institution 2; approximately 27% of the women of population 1 were categorized with diabetes. Overweight and obesity was positively related to blood pressure (r = 0.0003, p = 0.4173) and basal caloric expenditure (r=0.5215, p<0.0001). Higher levels of physical activity and higher angular phase values were correlated with muscle mass percentages (r=0.9529, p<0.0001) and (r=0.5990, p<0.0001) respectively. The body fat values were positively correlated with people’s age (r=0.37698, p=0.0011). Higher cell quality values were associated with higher body water content (r = 0.43855, p = 0.0001), muscle mass (r=0.65086, p<0.0001) and lower percentages of body fat (r=-0.4604 p<0.0001), decreasing as the age of the participants increased (r=-0.3298 p=0.0047).
Keywords: physical activity, diabetes, angular phase, hypertension, visceral fat, cardiometabolic risk
Honduras has a population of 8,143,564 inhabitants and population growth of 2.1%; 50.7% of the population are women and 49.3% are men.1 It is a lower-middle income country with 66.2% of Hondurans living in poverty (21.0% in relative poverty and 45.20% in extreme poverty). 55.5% of the population lives in rural areas and 44.5% in urban areas, and the most densely populated cities are Tegucigalpa and San Pedro Sula, with high levels of urban poverty.2 People are predominantly mestizo with the addition of eight ethnic groups, representing 7% of the population (440,000 people).3 Documents of the Pan-American Health Organization (PAHO) mention up to one million descendants of indigenous and Afro-Americans in the country.4
Honduras is undergoing an epidemiological transition characterized by an increase in noncommunicable diseases, among which obesity is one of the main ones.5 This is defined as a multifactor noncommunicable chronic disease epidemic, characterized by an increase in body weight at the expense of total body fat, which is also considered as the main global health problem.6 According to the PAHO, in Honduras regarding people 20 years and older, 34% are overweight and 21% present some degree of obesity, while in young populations of 13 to 15, 18.7% are overweight and 5.4% are obese.7
According to the Institute of Nutrition of Central America and Panama (INCAP), the products consumed by more than 90% of the Honduran population in both rural and urban areas and at different poverty levels include eggs, rice, beans, sugar and salt; less than 50% of the population reported consumption of beef and in 50% or more of the households, independent of the poverty level, the consumption of the following products was reported: sugar, eggs, beans, salt, rice, cheeses, poultry, bananas, onions, sauces, tomatoes, citrus fruits, sweet bread, potatoes/root crops and shortening. However, according to INCAP, food diversification is not the same in all areas of the country and is lower in rural areas.8
Metabolic syndrome (MS) has become one of the most rapidly expanding diseases in the last 15 years,9 characterized by a combination of risk factors in the same individual; the accepted characteristic elements of this syndrome: abdominal obesity, atherogenic dyslipidemia, high blood pressure, insulin resistance and/or glucose intolerance, prothrombotic and proinflammatory states.10 Honduras does not have prevalence studies on MS; however, the study conducted by Hall et al. in the city of El Progreso, Yoro, reported that 49.6% of the general population and 70.5% of hypertensive patients had MS,11 and in the outpatient department of internal medicine of the National University Hospital, it was found that 62.5% of hypertensive patients, 60% of diabetics and 98% of those suffering from both diseases developed MS;12 this represents a significant public health problem.
The fat and lean components of the body, including the percentage of visceral fat, muscle mass, body water and quality of cellular life are closely linked to obesity, aging and chronic diseases with subsequent morbidity and mortality.13 Recent technological progress allows the quick and accurate evaluation of the global body composition thanks to bioelectrical impedance. The primary objective of the study was to evaluate the metabolic state through the values of hemoglobin, cholesterol, blood glucose and blood pressure and its correlation with the percentage of visceral fat, muscle mass, body water, and cellular quality of life in two Honduran populations that are actively employed.
This study was made possible by the financial support of the Institute for Technology in Health Care (ITHC).
Type of study, sample estimation and data analysis: A descriptive cross-sectional prevalence study was carried out; sampling was non-probabilistic, voluntary, with inclusion and exclusion criteria, and aimed at working people from two different institutions with a sample from institution 1 of 52 people (population 1), and 20 people from institution 2 (population 2). The data were analyzed using descriptive statistics and data correlation using Statistical Analysis Software 9.4.
Informed consent: Participants in the study were informed about its objectives and their responsibilities and rights through informed consent. All data was handled with absolute confidentiality.
Data collection: The data collection was carried out in the Human Nutrition Laboratory of the Food Science and Technology Department at the Panamerican Agricultural School, Zamorano University in Honduras.
Clinical history and sociodemographic survey: A survey was given to compile the participants’ personal data, family backgrounds of chronic noncommunicable diseases (CND) and economic income. Information was also collected about chronic hereditary diseases and alcohol and tobacco consumption habits.
Biochemical measurements: The results of the biochemical tests (hemoglobin, cholesterol and glucose) were obtained with portable equipment, extracting a drop of blood from a finger through a lancet and using specific equipment for the different biochemical analyses. The equipment used in this study uses the technique of reflectometry with capillary blood, accepted for epidemiological studies because it is less painful and expensive, increasing its accessibility for the general population.14 The equipment used and parameters for each test are detailed below:
Hemoglobin: a HemoCue Hb 201+ portable analyzer was used, and the results were obtained in units of g/dL. These were compared with the "Concentrations of hemoglobin to diagnose anemia," according to WHO criteria15 in Table 1.
|
Sex |
Diagnostic |
Hemoglobin (mg/dL) |
|
Without anemia |
>12 |
|
|
Male |
Anemia low |
11–11.9 |
|
Anemia moderate |
8–10.9 |
|
|
Anemia grave |
<8 |
|
|
Female |
Without anemia |
>13 |
|
Anemia low |
10–2.9 |
|
|
Anemia moderate |
8–9.9 |
|
|
Anemia grave |
<8 |
Table 1 WHO parameters to diagnose anemia by sex according to hemoglobin levels, 2011
Source: WHO 2011.
Cholesterol: for the analysis of total cholesterol, a Coker® brand Accutrend® Plus portable cholesterol tester was used, and the results were obtained in units of mg/dL and were compared with the parameters of the National Heart, Lung and Blood Institute16 in Table 2.
|
Diagnostic |
Total Cholesterol (mg/dL) |
|
Normal |
<200 |
|
High Limit |
200–239 |
|
High |
≥ 240 |
Table 2 Parameters of the National Heart, Lung, and Blood Institute to diagnose total cholesterol levels in adults 2005
Source: National Heart, Lung and Blood Institute 2005.
Glycaemia: Fasting glucose levels were analyzed with an Aviva Plus Accu-Chek® glucometer, results were obtained in units of mg/dL and compared with the "Glucose concentrations to identify impaired glucose" of the National Diabetes Institute and Digestive and Kidney Diseases17 in Table 3.
|
Diagnostic |
Plasmatic Glucose (mg/dL) |
|
Normal |
<99 |
|
Pre-diabetes |
100–125 |
|
Diabetes |
>126 |
Table 3 Parameters of the National Institute of Diabetes and Digestive and Kidney Diseases to diagnose diabetes in adults 2016
Source: National Institute of Diabetes and Digestive and Kidney Diseases 2016.
Blood pressure: Blood pressure was measured with an OMRON Model HEM-7121-E automatic digital blood pressure monitor. The systolic and diastolic blood pressure in mmHg was compared with the parameters established by the American Heart Association,18 in Table 4.
|
Diagnostic |
Systolic Pressure (mmHg) |
Diastolic Pressure (mmHg) |
|
Normal |
<120 |
<80 |
|
Pre-hypertension |
120–139 |
80–89 |
|
Hypertension |
>140 |
>90 |
Table 4 Parameters of the American Heart Association to diagnose blood pressure levels in adults 2016
Source: American Heart Association 2016.
Anthropometric measurements: For the measurement of weight, height and body mass index (BMI), the SECA® brand "Medical Body Composition Analyzer 514" (mBCA) was used. The level of obesity was established according to the parameters set by the World Health Organization19 presented in Table 5.
|
Classification |
IMC (kg/ m2) |
|
|
Low weight |
<18.5 |
|
|
Normal weight |
18.5 – 4.9 |
|
|
Overweight |
25.0 – 9.9 |
|
|
Obesity |
30.0 – 9.9 |
|
|
Extreme Obesity |
>40 |
|
Table 5 WHO classification for nutritional status according to the 2016 Body Mass Index
Source: WHO 2016.
Percentage of visceral fat, muscular mass and cellular quality of life: These variables were determined by the mBCA 514 comparing the results obtained with a gold standard. Table 6 presents the criteria for the diagnosis of the participants’ visceral fat.20
|
Sex |
Diagnostic |
Percentage of Visceral Fat |
|
Male |
Normal |
1.5–2.7 |
|
High |
>2.8 |
|
|
Female |
Normal |
0.8–1.7 |
|
|
High |
>1.8 |
Table 6 Criteria for determining the level of visceral fat according to SECA 2000
Source: SECA 2000.
Muscular mass: The reference percentages for muscular mass used for this study were 60 to 72.5% for women and 70% to 84% for men pursuant to TANITA 2016.21
Percentage of water: The reference range used for percentages of water in healthy people were from 45 to 60% for women and from 50 to 65% for men pursuant to TANITA 2016 (Table 7).22
|
Classification |
(GET/GER) |
|
1.0-1.4 |
Sedentary |
|
1.5-1.6 |
Low active |
|
1.6-1.9 |
Active |
|
1.9-2.5 |
Very Active |
Table 7 Criteria to physical activity levels according to Onzari 2016
Source: Onzari 2016.
Quality of cellular life: In terms of cellular quality, Barbosa-Silva et al.,23 conducted an evaluation to estimate angular phase values in healthy individuals to provide reference values, according to their results, optimal cell quality values are: 6.93±1.15 for men and 6.53±1.01 for women.
Determination of physical activity level: The participants’ physical activity levels were determined through the parameters established by Onzari24 as the ratio between total energy expenditure and that at rest.
Determination of metabolic syndrome: The status of metabolic syndrome was determined by the parameters established by the Latin American group of the International Lipid Information Office (ILIB LA), which states that a person presents metabolic syndrome if the sum of the points is equal to or greater than three, based on the score attributed to each of the risk factors.25 These criteria are shown in Table 8.
|
Factors of Risk |
Values of reference |
Score |
|
Glucose |
Gl fasting ≥ 110 mg/dl |
2pts |
|
Hypertension Arterial |
≥ 130/85mm Hg |
1pt |
|
HighTriglycerides |
≥ 150mg/dl |
1pt |
|
C-HDL |
< 50mg/dl Male |
1pt |
|
< 50mg/dl Female |
1pt |
|
|
Abdominal Obesity |
IMC > 30kg/m2 |
1pt |
Table 8 Criteria for determining metabolic syndrome ILI B LA 2009
Source: Torresani and Somoza 2009.
Characterization of the population
Population 1 was composed of 52 people (30 women and 22 men), while population 2 was composed of 20 people (11 women and 9 men). The results of all the variables evaluated and correlated, as well as the statistical analysis of comparison of averages for the two female populations and are presented in Table 9.
|
Female |
Population 1 |
Population 2 |
||
|
Variable |
Average±ED |
CV(%) |
Average±ED |
CV (%) |
|
Stature (m) |
1.57±9.01a |
20.25 |
1.56±0.06a |
4.03 |
|
Weight (Kg) |
68.28±11.81a |
17.3 |
68.74±12.83a |
18.67 |
|
IMC (kg/m2) |
27.81±4.94a |
17.76 |
28.098±4.42a |
15.71 |
|
Glucose (mg/dl) |
113.69±23.95a |
21.06 |
93.90±9.00b |
9.58 |
|
Hemoglobin (mg/dl) |
12.50±1.44a |
11.55 |
12.41±1.00a |
8.06 |
|
Systolic pressure (mmHg) |
121.89±21.34a |
17.51 |
124.90±11.99a |
9.6 |
|
Diastolic pressure (mmHg) |
76.22±12.66a |
16.61 |
78.63±6.15a |
7.83 |
|
Cholesterol (mg/dl) |
190.18±41.22a |
21.67 |
194.00±18.67a |
9.63 |
|
Total fat (%) |
40.16±5.63a |
14.34 |
38.40±6.49a |
16.91 |
|
Visceral Fat (%) |
2.40±0.79a |
32.71 |
1.95±0.98a |
50.17 |
|
Muscle (kg) |
25.61±11.17a |
43.6 |
18.30±2.96a |
16.15 |
|
Corporal water (%) |
59.67±18.88a |
31.63 |
45.40±5.04a |
11.09 |
|
Cellular quality (φ) |
5.24±0.55a |
10.5 |
5.30±0.32a |
5.98 |
Table 9 Comparison analysis of averages of the results of women from both populations
a-b, Averages with different letters in the same row indicate significant differences between populations (P <0.05).
The women of population 1 and 2 did not present a significant difference in the variables evaluated except in the glucose content, with population 1 having higher values (113.69±23.95 mg/dL). The results of all the variables evaluated and correlated, as well as the statistical analysis of comparison of averages for the two male populations are presented in Table 10. The men of both populations did not present significant statistical differences (p>0.05) in the variables evaluated except in the diastolic pressure (p <0.05), with male population 2 having higher values (88.33±10.73mmHg).
|
Male |
Population 1 |
Population 2 |
||
|
Variable |
Average±ED |
CV(%) |
Average±ED |
CV (%) |
|
Stature (m) |
1.69±0.07a |
4.2 |
1.68±0.04a |
2.57 |
|
Weight (Kg) |
83.10±10.68a |
12.9 |
88.57±11.26a |
12.71 |
|
IMC (kg/m2) |
28.74±3.10a |
10.79 |
31.17±2.91a |
9.34 |
|
Glucose (mg/dl) |
111.80±24.56a |
21.97 |
118.55±43.70a |
36.86 |
|
Hemoglobin (mg/dl) |
14.46±1.85 a |
99.17 |
14.94±1.05a |
7.03 |
|
Systolic pressure (mmHg) |
122.48±10.92a |
8.91 |
131.33±14.06a |
10.71 |
|
Diastolic pressure (mmHg) |
79.28±8.12b |
10.24 |
88.33±10.73 a |
12.15 |
|
Cholesterol (mg/dl) |
199.92±36.70a |
18.67 |
172.00±17.58 a |
10.22 |
|
Total fat (%) |
30.08±5.22a |
17.35 |
32.44±2.87a |
8.85 |
|
Visceral Fat (%) |
4.03±1.48a |
36.66 |
4.47±1.15 a |
25.59 |
|
Muscle (kg) |
34.09±13.78a |
40.4 |
28.86±3.18 a |
11.01 |
|
Corporal water (%) |
55.09±9.18 a |
16.65 |
49.21±1.88 a |
3.82 |
|
Cellular quality (φ) |
6.11±0.61 a |
9.92 |
6.40±0.53 a |
8.24 |
Table 10 Results of variables analyzed for men
a-b, Averages with different letters in the same row indicate significant differences between populations (P <0.05).
Age: The average in years for women and men of population 1 was 45.46±8.78 and 42.23±11.01 respectively, while for population 2 it was 43±10.84 and 41.55±10.1 respectively. Figure 1 shows the age frequencies for the populations of women and men of both populations. The population was stratified by age group, where the majority of women in population 1 are between 41 and 50 years old, while for the participants in population 2, the majorities are between 41 and 60 years old. For men, 7% of population 1 was between 31 and 40; whereas for population 2, 3% are between 31 and 40 years old.
Metabolic syndrome: 19.23% of population 1 was diagnosed with metabolic syndrome, 3.84% were men and 15.38% were women. While for population 2 15% was diagnosed with metabolic syndrome and in this case, all were men. The predisposing factors in the determination of the syndrome were the glucose values because they presented a higher value in the score established by said methodology.
Hemoglobin: According to the World Health Organization, anemia affects 1.62 million people globally, corresponding to 24.8% of the world population.26 In this study of women, 60% from population 1 and 73% from population 2 presented mild anemia. In men in population 1, 5% had mild anemia, 5% moderate anemia and 5% severe anemia. Noman from population 2 was found to have anemia.
Studies have shown that there is an association between the prevalence of anemia and Chronic Renal Failure (CRF), so when hemoglobin levels are less than 12 mg/dl in adult men and postmenopausal women, anemia promotes the development of this disease.27 The loss of ovarian function occurs at 48±5 years of age, which is why women with higher ages can be considered postmenopausal.28 15% of male employees in population 1 had hemoglobin levels less than 12 mg/dl and in the case of women 26% of population 1 and 36% of population 2 are in sufficient age ranges to be considered postmenopausal, increasing the risk in the female population of developing CRF.
Women from population 1 presented 12.50±1.44 mg/dL of hemoglobin while those from population 2 had 12.41±1.00 mg/dL, men presented values of 14.46±1.85 mg/dL for population 1 and 14.94±1.05 mg/dL for population 2. Finally, men in both populations presented optimal levels of hemoglobin (Figure 2).
Higher levels of hemoglobin were positively correlated with higher percentages of visceral fat (r =0.4023, p=0.033). This association was also found in an adolescent population in Brazil (r=0.258, p=0.024)28,29 and a study with the general population in Okinawa, Japan.30 It has been shown that hemoglobin and other serum components are correlated with visceral fat in adults with type 2 diabetes mellitus.31
Hemoglobin levels also had a positive correlation with muscle mass (r=0.4682, p<0.0001) showing that anemia is also related to lower muscle density; lower levels of hemoglobin could accelerate the modifications in muscle mass related to age,32 which leads to a loss of physical performance. Employees with a diagnosis of anemia are also at risk of suffering losses in muscle mass that could be further aggravated by aging related to cell quality. The hemoglobin values were positively related to cell quality (r=0.53961, p<0.0001) indicating that higher hemoglobin values indicate higher cell quality. Finally, these values had a negative correlation with body fat values (r=-0.2515, p=0.033); the lower the value of hemoglobin the higher the value of body fat.
Cholesterol: It has been established that elevated total serum cholesterol is associated with an increase in all causes of cardiovascular mortality in middle-aged adults.33 A study conducted to evaluate total cholesterol in populations of seven countries found that there is a linear relationship between cholesterol and mortality from coronary heart disease, indicating that an increase of approximately 20 mg/dL in cholesterol levels leads to an increase of 12% in the risk of mortality due to coronary diseases.34
In the present study, the mean cholesterol in the case of women was 194.00±18.67mg/dL and 190.18±41.22mg/dL for populations 1 and 2 respectively. For the male population, the mean was 199.92±36.70mg/dL and 172.00±17.58mg/dL for populations 1 and 2 respectively. For both sexes, population 1 had higher cholesterol values. In Figure 3 it can be seen that 30% of the women in population 1 were at the high limit level and 10% at the high level, while for population 2, 18% were at the high limit level. In the case of men, for population 1 18% was found at the high limit and 14% at a high level and for population 2 14% at the high limit. Therefore, 40% of women and 32% of men in population 1 have cholesterol problems, predisposing them to the development of other diseases that aggravate their health status
In addition, higher levels of total cholesterol were correlated with older people (r=0.2643, p=0.0248) indicating that as the age of both populations increases, cholesterol values will also tend to increase. The increase in low-density lipoprotein (LDL) production rates, as well as the decrease in fractional HDL elimination rates, are responsible for the increase in blood cholesterol levels with age.35
Glucose: Diabetes is considered one of the most frequent metabolic disorders in the world with increased prevalence in adults in recent decades.36 Urbanization has caused dramatic changes in lifestyles. In 2013, 382 million people had diabetes, and this is expected to increase to 592 million in 2035;37 therefore, establishing prevalence levels in populations at metabolic risk is essential for therapeutic interventions.
Women in both populations presented average cholesterol of 113.69±23.95mg/dl and 93.90±9.00mg/dl respectively. The male population presented averages of 111.80±24.56mg/dl for the population 1 and 118.55±43.70mg/dl for population 2. 53% of the women in population 1 and 27% of population 2 presented values high enough to consider them with pre-diabetes, while in population 1, 27% and are considered diabetic. 64% of men in population 1 and 33% of those in population 2 were identified with pre-diabetes, while 22% of population 2 is considered diabetic (Figure 4). These results require both populations to change lifestyles in order to reduce sugar consumption in their diets do exercise.
As a result of the short and long-term implications of failure to detect diabetes, several studies indicate that obesity and weight gain are associated with an increased risk of diabetes,38 and, that intentional weight loss reduces the risk of overweight people developing diabetes. Insulin resistance (caused by excess fat and physical inactivity) increases the probability of developing pre-diabetes, although this condition alone does not cause type 2 diabetes, it often favors the development of the disease by presenting a high demand in insulin-producing cells.39 Studies show40 that after exercise, muscle fibers have increased susceptibility to the action of insulin, thus avoiding the development of insulin resistance and reducing or maintaining glucose levels in the blood. Exercise physiology demonstrates that physical activity improves muscle uptake of glucose with no need for insulin. The more muscular myofibrils are present in the body, the greater amounts of glucose can be used to modify blood glucose levels.
Hypertension: The average systolic pressure values (mmHg) of women in populations 1 and 2 were 121.89±21.34 and 124.90±11.99 and diastolic pressure (mmHg) were 76.22±12.66 and 78.63±6.15 respectively. For men, averages of systolic pressure (mmHg) in populations 1 and 2 were 122.48±10.92 and 131.33±14.06 and diastolic pressure (mmHg) were 79.28±8.12 and 88.33±10.73 respectively. For both cases, population 2 presented the largest values in both the systole and diastole.
53% of women and 34% of men of population 1 and 36% of women and 44% of men of population 2 presented prehypertension. 16% of women and 19% of men of population 1 and 9% of women and 45% of men of population 2 were diagnosed with hypertension. In normal situations, variation in systolic blood pressure is considered an important risk factor for cardiovascular disease in people over 50 who are predisposed. However, having both systolic and diastolic arterial pressure high is a useful indicator at the time of diagnosing altered blood pressure. The risk of death from ischemic heart disease and stroke doubles with increasing systolic 20mmHg or 10mmHg diastolic in people between people 40 and 89.41 In this study, a higher percentage of females was identified with high blood pressure (Figures 5–7).
Systolic blood pressure was greater in overweight and obese people (P.=0.4173, a=0.0003) because systolic pressure is associated with increased body weight.42 Prehypertension and hypertension are often more prevalent among overweight and obese subjects.43 It has been shown that obesity can affect or modify secretion and/or use of different hormones in the body. The renin-angiotensin-aldosterone system, which seems to be most affected, is responsible for controlling blood volume in conjunction with the sympathetic nervous system, which is responsible for control sodium levels and body water.44 High blood pressure values are also associated with basal caloric expenditure (P.=.43817, a=0.0001), and thus, increases or decreases in pressure values will have a similar impact on the values of the basal caloric expenditure of the people evaluated.
Caloric expenditure and physical activity: Basal metabolism is defined as the caloric expenditure necessary to maintain the vital functions of the body in a state of rest. With respect to the distribution by sex; men require 1 kcal/kilo/hour and women 0.9/kcal/kilo/hour,45 and therefore, an increase in body weight (kilos) involves an increase in baseline caloric expenditure.
The BMI was also linked to a higher basal caloric expenditure and higher blood pressure, r=0.52153 p<0.0001 and r=0.24949 p=0.0001 respectively. The specific mechanisms by which physical activity can reduce blood pressure and prevent the development of hypertension are not clearly defined, largely because hypertension is a disease of multifactorial etiology,46 and it is unknown to some extent how several factors (including obesity) can interact to contribute to the development of hypertension. However, several mechanisms have been proposed to explain this relationship, recent findings in animal studies suggest that aerobic exercise favors through beneficial alterations in insulin sensitivity and autonomic nervous system function;47 resistance training could influence through beneficial alterations in vasoconstrictor regulation according to studies with laboratory rats.48 Although there is no consensus on the mechanisms of action, the existing evidence is incontrovertible, physical activity contributes to the primary and secondary prevention of several chronic diseases and is associated with a lower risk of premature death.49 Higher levels of physical activity were correlated with higher percentages of muscle mass (r=0.95202, p<0.0001) and higher values of angular phase (r=0.59901, p<0.0001). An analysis of the relationship between physical activity and muscle mass has shown that continuous physical activity from middle age to old age could be one of the most important factors for the prevention of sarcopenia (loss of muscle mass) during old age.50
Body mass index (BMI): Since 2013 the American Medical Association, among many others, has adopted a more defined policy to categorically consider obesity as a disease.51 Obesity is the cause of serious health complications such as coronary heart disease, diabetes, kidney and liver diseases and even cancer, therefore, public health institutions must actively focus their efforts on combating this problem. For these purposes, the BMI continues to be the benchmark for diagnosing levels of obesity in populations, as it is a parameter that allows to quickly identify the nutritional status of populations. Studies similar to this in other population groups have been developed previously in population 1, evaluating the BMI in students and employees.52–54
Women presented an average BMI for populations 1 of 27.81±4.94 and 28,098±4.42 for population 2, and men of 28.74±3.10 and 31.17±2.91 respectively. For both sexes, population 2 presented higher BMI values, which was reflected in greater weight problems. 40% of women in population 1 are overweight, 20% are obese and 7% have extreme obesity, while in the case of population 2, 45% of the population was overweight and 28% were obese. 55% of men in population 1 were overweight and 31% were obese, while for population 2, 33% were overweight and 67% were obese.
Although BMI is often considered an indicator for the diagnosis of nutritional status, it is no longer considered a feasible tool when evaluating body fat, given that this indicator includes liquids, total body fat, muscle, organs and skin; to that end the Center for Disease Control and Prevention55 considers that the BMI should be used to estimate the weight status of a population in order to identify possible weight alterations, and not as a direct tool for the measurement of body fat. Thus, research suggests the use of other techniques to estimate body fat such as: the thickness of skin folds, bioelectrical impedance and others.
Figures 8 & 9 show the prevalence of overweight and obesity in the two populations according to age groups. In the case of population 1, the prevalence of overweight was higher in all ages except in the range of 61-70 years; obesity increases in the range of 31-40 and remains constant in the range of 41-50 to later become more prevalent in the range of 51-60 years. In the case of population 2, overweight remains constant in the first three age ranges and then decreases in the range of 51-60 and remains constant in the range of 61-70 years. Obesity becomes more prevalent in the range of 31-40 and finally decreases in the ranges of 51-60 and 61-70 years.
Body fat: There are still no defined ranges in normal populations regarding the amount of healthy body fat universally accepted, but there are empirically established limits. Most knowledge about health risks related to obesity is based on its association with the BMI and not directly with the amount of fat.
Models have been proposed to develop guides based on BMI56 with the objective of establishing a precedent in terms of ranges of healthy body fat percentages due to inconsistencies inherent to the BMI classifications. Some people who are classified as overweight do not have excess fat, others instead have a BMI within the normal range and yet a high percentage of fat in their body weight. Measurements such as the percentage of body fat could complement the BMI to properly estimate obesity and the risk of related diseases once these ranges are clinically developed.
Unfortunately, there is still no consensus on how body fat is related to morbidity and mortality due to the absence of appropriate prospective studies. Figure 10 shows the relationship between age and body fat percentage, identifying a clear increasing trend in body fat as people increase in age. This pattern was the same for the four groups evaluated.
On the other hand, the relationship between BMI and body fat percentage has been studied in several ethnic groups.57 in order to estimate the capacity of the BMI to predict adiposity. In this study, both variables were positively correlated (r=0.4936, p <0.0001). The average body fat for women was 40.16±5.63% for population 1 and 38.40±6.49% for population 2, while the average for men was 30.08±5.22% for population 1 and 32.44±2.87% for population 2.
The values of body fat correlated positively with the age of the people (r=0.37698, p=0.0011), indicating, as is demonstrated in Figure 10, that for the four groups evaluated as the age of the persons increased, the percentage of body fat also increased. The values also showed a high negative correlation for the body water content (r=-0.95264, p <0.0001), showing an inverse relationship, that is, as the body fat content increases, the water content decreases.
Visceral fat: Excess fat located in the abdomen and around the waist is associated with an even greater risk of diseases linked to obesity. Scientists used to think that fatty tissue functioned only as energy storage. However, studies have shown that visceral fat is considered to be the major precursor of hormones and pro-inflammatory substances that cause various health problems such as insulin resistance, high blood pressure, unbalanced cholesterol and cardiovascular disease.58
13% of women from population 1 and 55% from population 2, as well as 14% of men from population 1 and 11% from population 2, presented android type abdominal fat. The averages of women from populations 1 and 2 were 2.40±0.79% and 1.95±0.98% respectively, while men from population 1 presented 4.03±1.48% and 4.47±1.15% in population 2. Women from the population 1 had higher values of visceral fat, while in population 2 it was the men. Individuals genetically predisposed to accumulate a higher percentage of fat in the mid-waist area (android type) tend to have a health condition more disadvantaged than those who accumulate higher percentages of fat in the area of the thighs (gynecoid type).58 A higher percentage of visceral fat was associated with overweight and obesity (r=0.67122, p <0.0001). The results can be seen in Figure 11.
Muscle mass: Estimating the proportion of muscle in adults allows us to understand the loss of muscle mass involved in aging; a situation that also leads to a decrease in the ability to perform physical activity.59 Greater amounts of body muscle do not equal greater muscle strength, as maintaining or gaining muscle mass does not prevent the reduction of muscle strength associated with aging.60,61
The average muscle for women in population 1 was 25.61±11.17kg and 18.30±2.96kg for those belonging to population 2. In the case of men, the averages for populations 1 and 2 were 34.09±13.78kg and 28.86±3.18 respectively. Both sexes of population 1 had a high muscle value. High values of muscle mass showed a positive correlation with the angular phase (r=0.65086 p<0.0001), indicating that the higher the muscle mass values, the better the cellular quality of the people. On the other hand, muscle mass also presented negative correlations with age and body fat r= -0.25881 p=0.0281 and r=-0.45051 p<0.0001 respectively. The higher the age of the people evaluated, the lower the values of muscle mass, and the higher the values of muscle mass, the lower the values of body fat.
Body water: Blood indices and urinary indicators are usually used to determine the levels of hydration. The determination of body water by averages of bioimpedance analysis has allowed toobtain faster and more accurate results. The values for body water enable to evaluate the nutritional status in people with chronic renal failure.62
The average body water for women in population 1 was 59.67±18.88% and 45.40±5.04% for those belonging to population 2. In the case of men, the averages for populations 1 and 2 were 55.09±9.18% and 49.21±1.88% respectively. Both sexes of population 1 presented high percentages of body water. In the case of population 1, in all the nutritional states identified, the percentage of muscle and body water were prevalent except in the state of extreme obesity where the amount of body water decreased, but the percentage of body fat increased. For the case of population 2 in the three nutritional states identified, the content of body muscle and body water were prevalent. Body water values were negatively correlated with age and BMI of the individuals r=-0.2998, p=0.0105 and r=-0.58042, p <0.0001 respectively. The higher the age of the people, the lower the values of body water, and the higher the water content in people, the lower their BMI value. The results are shown in Figures 12 & 13.

Figure 12 Relationship between the body mass index and percentages of fat, muscle and body water – population 1.
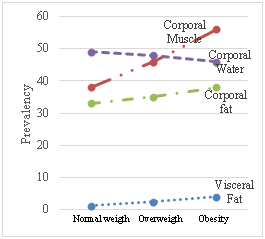
Figure 13 Relationship between the body mass index and percentages of fat, muscle and body water - population 2.
Cell quality: The phase angle has been interpreted as an indicator of the integrity of the cell membrane.63 Other studies have used the phase angle to predict body cell mass, emphasizing its importance as a nutritional indicator.64–66 The phase angle has been studied even as an indicator with predictive capacity, finding a positive correlation between phase angle and survival in patients with lung cancer,67 HIV,68 hemodialysis,69 critical condition patients70 and septic patients.71
The average angular phase for women in population 1 was 5.24±0.55 and 5.30±0.32 for those in population 2. In the case of men, the averages for the two populations were 6.11±0.61 and 6.40±0.53 respectively. Both sexes of population 2 presented a high percentage of body water. Figure 14 shows that for men in population 2 and women in population 1, the angular phase tends to remain constant or in similar values as the age of the individuals increases, while in the case of women of population 2 and men of population 1 the angular phase tends to decrease as the age of the persons evaluated increases. Higher cell quality values are related to healthier levels of hemoglobin (r=0.53961, p <0.0001), with a higher body water content (0.43855 p=0.0001), more muscle mass (r=0.65086, p <0.0001) and lower percentages of body fat (r=-0.4604 p <0.0001). The cellular quality decreased as the age of the participants increased (r=-0.3298 p=0.0047); this trend can be observed in Figure 14.
Social situations, low resources for the financing of the present study, the population was included (N=72) participants being this the main limitation, consequence of this we cannot give significant conclusions.
Overweight and obesity are considered a nutritional emergency with manifestations in both populations in different age ranges, making them a prevalent disease. The cellular and life quality of people is negatively affected by the content of cholesterol and total and visceral fat and is positively affected by high values of hemoglobin and body water. Metabolic syndrome was diagnosed in 19.23% of population 1, with a higher prevalence in the women, while in population 2, 15% had MS but with prevalence only in the men. The risk factor with the highest prevalence in the study population, involved blood glucose (p=>0.05) values followed by the degree of overweight/obesity (p=0.09) and blood pressure systolic (p=0.417).
None.
The authors declare that they have no conflict of interest.

©2019 lorio, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.