Journal of
eISSN: 2378-3184


Research Article Volume 8 Issue 5
Department of Marine Biology, University of Basrah, Iraq
Correspondence: Shaker G Ajeel, Department of Marine Biology, Marine Science Centre, University of Basrah, Iraq
Received: September 13, 2019 | Published: October 25, 2019
Citation: Shaker GA, Mohammad FA, Dawood SA. Abundance and diversity of zooplankton in the Tigris River Northern of Basrah, Iraq. J Aquac Mar Biol . 2019;8(5):171-178. DOI: 10.15406/jamb.2019.08.00258
Seasonally variations of the quality and quantity of zooplankton were studied in two stations in the terminal sector of Tigris River and one station at the confluence of the Tigris and Euphrates area, in Al-Qurnah North of Basrah City, during October 2015 to August 2016. Samples of zooplankton were collected by plankton net (100µm. Mesh size). Salinity changed from 1% at St. 2 to 1.8% at St. 3, the pH varied from 7.5–8.2 and the dissolved oxygen from 6mg/l to 8.7 mg/l at St. 3 and St. 1 respectively. In study area the population density of zooplankton ranged between 20.3ind./m³ during Autumn and 243.41ind./m³ during Winter at station 1 (Al-Jewaber Bridge). The results showed that the Crustaceans were the dominated group that comprised 92.9%, 93.1% and 98.1% in study area respectively. Copepoda were the dominant in three stations, which constituted about 43.8% followed by Cladocera 35.2%, Cirripede larvae 7.2%, and Zoea of shrimp 4.2 % of the total zooplankton respectively. Maximum richness (D) of (1.66) was obtained at station 1 during summer and autumn and higher Diversity (H) of (1.41) was recorded at station 1 during spring, while higher evenness (J) of (0.70) was obtained at station 2 during summer. The Jaccard׳s index values were close and revealed a higher similarity between stations 1 and 2, and lower values between stations 1 and 3.
Keywords: zooplankton, abundance, diversity Tigris River, North of Basrah
Zooplankton are important components of food webs in aquatic ecosystems throughout the world, channeling energy and nutrients from algae and bacteria to fish and other aquatic animals. Because they are highly productive and important in fish diets, an improved understanding of zooplankton production and growth can be applied to increase fish production in aquaculture facilities and in the aquatic environments. Therefore, the interest has been focused here on this important group, as it has not been well documented.
However, the research on the zooplankton of Basrah extends back to Gurney1 who for the first time surveyed freshwater Crustaceans of the lower Mesopotamia. In Basra the freshwater bodies include; Shatt Al-Arab River, Garmat-Ali River, Shatt Al-Basrah Canal and the marshes. The studies of zooplankton at Shatt Al-Arab River include; Salman et al.2 investigated the monthly changes of the zooplankton from 1982–1984, AL-Zubaidi and Salman3 and Ajeel et al.4 study zooplankton in Central and Southern of Shatt Al-Arab. Moreover, Ajeel5 and Ajeel6 studyabundance and distribution of the zooplankton in Shatt Al-Arab, Shatt Al-Basrah and Khour Al-Zubair Channels. Later, Ajeel7 studied seasonal variations of zooplankton abundance in Shatt Al-Arab River. While, Ajeel et al.8 surveyed the zooplankton of Garmat-Ali River. At the Basrah Marshes Southern Iraq AL-Saboonchi et al.9 studied the zooplankton(near Garmat-Ali River), qualitatively and quantitatively, between 1980 and 1981, Ajeel et al.10 studied the seasonal abundance of zooplankton in the southern Iraqi Marshes and Ajeel et al.11 studied seasonal variations of zooplankton in Al-Hammar Marsh.
However, the earliest studies were mainly concerned with the taxonomy of Cladocera and to a lesser extent of Copepoda, whereas the latter articles were investigating the abundance and distribution of Cladocera and Copepoda and only few papers were concerned with Rotifera abundance. Therefore, there is no thorough investigation of the various groups of zooplankton throughout different stations in north Basrah and for one complete year. For this reason and for estimating the zooplankton production in various localities in Basrah, which has not been conducted before, the present study was carried out.
The study was carried out between October 2015 and August 2016 on a seasonally basis. Samples were taken from three stations south Tigris River, North of Basrah (Figure 1). The first station near the Al-Jewaber Bridge (31°0953ʹ 0.45״N and 47°2556ʹ 0.89״E), Second station near the Hamay on Bridge (31° 0748ʹ 0.15״ N and 47° 2638ʹ 0.79״ E) and the third station Shatt Al-Arab in Al-Qurna region (near the confluence of the Euphrates and Tigris) (31° 0042ʹ 71״ N and 47°2623ʹ 0.23״ E).
Sample collection
Zooplankton samples were collected seasonally from surface water by using a 100μm mesh-sized zooplankton conical tow net and having a mouth aperture of 40 cm in diameter. A digital flow meter was mounted in the middle of the mouth of the zooplankton net. The net was horizontally towed behind a boat running at its lowest speed for 10-15 minutes, and then collect the zooplankton that have been retained by the net. The reading of the flow meter was taken before and after towing. At each station, samples of zooplankton were collected, transferred to containers (plastic bottles). The plankton samples were immediately fixed in 4% formaldehyde.
Water temperatures were measured by a thermometer with 0.1ºC sensitivity. Salinity and pH measurements were performed by YSI 556MPS. Dissolved oxygen concentrations were measured by Winkler method. Turbidity were measured by HANNA instrument, Microprocessor Turbidity Meter HI 93703.
In the laboratory, samples were poured into a graduated vessel, and diluted if densely populated. Then a 10ml subsample was taken and placed in a Bogorov chamber, examined and counted under a dissecting microscope. This procedure was repeated for 3 times, and then the whole sample was examined for the rare species.
The volume of water was calculated using the method of De Bernardi.12
Where: V=volume of water filtered by the net and is measured in cubic meters, , r=half diameter of the net mouth aperture, (20cm), d = number of revolutions of the flow meter multiplied at 0.3.
Then the result was dividing by 10,000 to convert the result unit per cubic meter. The number of individuals were calculated in the sample diluted to 1000ml in the manner prescribed by APHA13 and expressed the result in cubic meter
Where: C=the number of individuals in the subsample
VI = volume of sample (ml).
VII = the size of the subsample (10ml).
VIII=volume of water filtered in cubic meters
Ecological Indices
Diversity index (H) Shannon Weaver
The diversity index (H) was calculate from the equation of Shannon-Weaver14 as follows:
Where:
ni=Number of members of the same species
N=The total number of individuals in the sample
Evenness (J)
Evenness (J) was calculated by the equation of Pielou15
Where:
H = Shannon Weaver diversity index
S = Number of species
Richness index (D)
Richness was calculated by the equation of Margalef16 as follows:
Where:
D=richness index
S=total number of species
N=total number of individuals
Jaccard's similarity index Ss%
Jaccard's similarity index Ss% was calculated according to Jaccard17, as follows: -
Where:
a-The number of species of Cladocera found in A and B samples.
b-The number of species of Cladocera found in sample B and not found in sample A.
C-The number of species of Cladocera found in sample A and not found in sample B.
Statistical analysis
The correlation coefficient between zooplankton and environmental and physical factors was calculated using statistical program Canoco (2004).
The zooplankton distribution varies both spatially and temporally according to the environmental conditions prevailing in the region. Differences may also arise due to the nature of distribution of the zooplankton, namely patchiness that may be the cause of the great variations in the catches of the nets.18 Moreover, the mesh-size of the net is an important factor controlling the quality and quantity of the catch.
Hydrographic of the stations
Water temperatures at three stations are very close to each other, it ranged between 9.5°C (in January 2016) at station 1 (Al-Jewaber Bridge) and 37.5°C (in Summer 2016) at station 3 (Shatt Al-Arab). Salinity changed from 1% at station 2 (Hamayon Bridge) to 1.8% at station 3. The pH varied from 7.5–8.2 and the dissolved oxygen from 6mg/l to 8.7mg/l at station 3 and station 1 respectively. While the highest value of turbidity 99.8 NTU were encountered during winter at station 1, whereas the lowest value 8.8 NTU were recorded during spring at station 3. Chlorophyll-a values ranged from 0.58mg/m3 during winter at stations 2 & 3 to 8.6mg/m3 during summer and spring at station 3, Table 1.
Seasons |
Stations |
W.T. |
Sal. |
PH |
D.O. |
Turbidity |
Chlorophyll a |
Autumn |
St. 1 |
23 |
1.12 |
7.9 |
6.8 |
97.8 |
2.98 |
St. 2 |
23 |
1.14 |
7.7 |
6.3 |
10.3 |
6.36 |
|
St. 3 |
24 |
1.19 |
7.8 |
6.0 |
16.9 |
2.60 |
|
|
|
|
|
|
|
|
|
Winter |
St. 1 |
19.5 |
1.35 |
7.8 |
8.7 |
99.8 |
2.02 |
St. 2 |
20 |
1.4 |
8.0 |
8.1 |
38.5 |
0.58 |
|
St. 3 |
21 |
1.4 |
7.7 |
7.6 |
27.7 |
0.58 |
|
|
|
|
|
|
|
|
|
Spring |
St. 1 |
24.5 |
1.07 |
8.2 |
8.0 |
35.3 |
2.89 |
St. 2 |
24.8 |
1.0 |
8.0 |
7.9 |
47.3 |
2.89 |
|
St. 3 |
25.8 |
1.1 |
7.8 |
7.6 |
8.83 |
8.6 |
|
|
|
|
|
|
|
|
|
Summer |
St. 1 |
37.4 |
1.4 |
7.8 |
6.6 |
56.8 |
2.89 |
St. 2 |
37.3 |
1.5 |
7.6 |
6.4 |
59.5 |
2.89 |
|
St. 3 |
37.5 |
1.8 |
7.5 |
6.1 |
9.9 |
8.6 |
Table 1 Water temperatures, Salinity, pH, dissolved oxygen, turbidity and Chlorophyll a at study stations during October 2015 to August 2016
The present results indicate that the density of zooplankton was few in comparison with other areas in Basra. It was found some differences in the abundance of zooplankton among the three stations sampled. This is probably due to the environmental conditions. It is obvious that the highest density of zooplankton recorded during the Winter 2016 as the peak density (243.4ind/m3) was reported in station 1 (Al-Jewaber Bridge), while the low density 20.3ind/m3 was reported in Autumn at the same station. Moreover Abbas et al.19 studied the abundance and distribution of Zooplankton in the northern sector of Shatt Al-Arab, they reported the zooplankton density it was ranged between (79-65170ind/m3), where Cirripede larvae dominated the zooplankton community at all the stations. Cladocera was second important group, followed by Copepoda. However, Salman et al.2 reported a density of zooplankton in Shatt Al-Arab, ranged between 21–642ind/m3 during May–December and January 1982/83 at Mhajran near the Basrah city center. This controversy is, perhaps, largely due to the difference in mesh-size of the net used, as the net used by the latter authors was 200µm. However, further downstream, at Al-Seba the density of zooplankton varied from 97– 13438Ind/m3, and two peaks of zooplankton abundance were found, one during summer and the other at the end of winter.3
The results showed the high densities of zooplankton were recorded during the winter at station 1 and 2 while at station 3 the high density were recorded during summer, and less density recorded during the autumn in three stations, and there is no effect of environmental factors on the density of the zooplankton. The differences of average density of zooplankton between three stations were few (110.8ind/m3), (74.3ind/m3) and (96.4ind/m3) respectively. The reason may be due to the nature of distribution of the zooplankton, namely patchiness that may be the cause of the great variations in the catches of the nets.18
In the study areas the seasonal variation of average density of zooplankton ranged between 51.3ind/m3 in Autumn to 148.1ind/m3 in Winter, Figure 2. While in study stations the average density of zooplankton ranged between 73.3ind/m3 at station 2 (Hamayon Bridge) to 142.4ind/m3 at station 3 (Shatt Al-Arab) Table 2. The average density in all stations was 118.7ind/m3. The Crustaceans was dominant in this area (95%), Copepods constitute 43.7%, the second important group was Cladocerans 35.3%, Cirripede larvae 7.2% Zoea of shrimp 4.1%, Amphipods 2.5% then Bivalve larvae 2.4% of the total zooplankton Figure 3. Cyclopoida of Copepoda exhibited the highest peak is reached 59.2ind/m3 in station 3, which comprised 39.9% of total zooplankton.
Zooplankton |
St. 1 |
St. 2 |
St. 3 |
Calanoida |
4.05 |
1.1 |
0.2 |
Cyclopoida |
49.4 |
33.4 |
59.2 |
Harpacticoida |
0.7 |
1.1 |
3.2 |
Nauplii larvae |
1.6 |
0.5 |
1.2 |
Total Copepoda |
55.9 |
36.1 |
64 |
Cladocera |
54.1 |
24.4 |
47 |
Insect larva |
0.2 |
0.6 |
2.3 |
Amphipoda |
7 |
1.9 |
0.2 |
Isopoda |
- |
- |
0.01 |
Ostracoda |
0.03 |
3 |
0.3 |
Cirripede larvae |
0.5 |
- |
25.2 |
Zoea of shrimp |
12.1 |
2.2 |
0.4 |
Zoea of crab |
0.7 |
- |
0.3 |
Total Crustaceans |
130.6 |
68.3 |
139.8 |
Rotifera |
0.3 |
4.4 |
2.2 |
Polychaete larvae |
1.2 |
0.6 |
0.3 |
Fish larvae |
- |
0.005 |
0.02 |
Bivalve larvae |
8.5 |
- |
- |
Gastropoda |
0.007 |
0.007 |
- |
Total Zooplankton |
140.6 |
73.3 |
142.4 |
Table 2 The average density of Zooplankton (ind./m3) at Study area
The population density of zooplankton ranged between 20.3ind/m3 in Autumn 2015 to 243.4ind/m3 in Winter 2016 Table 3. The average density was 140.6ind/m3. The Crustaceans was dominant in this area (92.9%), where their numbers ranged between 18.8ind/m3 in Autumn to 242.2ind/m3 during Winter. Total Copepoda constitute 39.7%. The second important group was Cladocera 38.4%, Zoea of shrimp 8.6% then Bivalve larvae 6% of the total zooplankton Figure 4. Cyclopoida of Copepoda exhibited the highest peak is reached in Summer (134ind/m3), which comprised 35.1% of total zooplankton and 88.3% of total Copepoda, while Cladocera exhibited a rise in Winter 2016 (211ind/m3).
Zooplankton |
Autumn |
Winter |
Spring |
Summer |
Calanoida |
0.6 |
8.1 |
1.8 |
5.7 |
Cyclopoida |
10.5 |
21 |
32 |
134 |
Harpacticoida |
1 |
0 |
0 |
2 |
Nauplii larvae |
5 |
0 |
1.2 |
0.6 |
Total Copepoda |
17.1 |
29.1 |
35 |
142.3 |
Cladocera |
1.3 |
211 |
3 |
1 |
Insect larva |
0.3 |
0.1 |
0.6 |
0.01 |
Amphipoda |
0 |
0 |
18 |
10 |
Ostracoda |
0.1 |
0 |
0.01 |
0.01 |
Cirripede larvae |
0 |
2 |
0 |
0 |
Zoea of shrimp |
0 |
0 |
14.2 |
34.3 |
Zoea of crab |
0 |
0 |
2 |
1 |
Total Crustacea |
18.8 |
242.2 |
72.81 |
188.62 |
Rotifera |
1.2 |
0.01 |
0 |
0 |
Polychaete larvae |
0.3 |
1.2 |
3.1 |
0.03 |
Bivalve larvae |
0 |
0 |
0 |
34 |
Gastropoda |
0 |
0 |
0 |
0.03 |
Total Zooplankton |
20.3 |
243.41 |
75.91 |
222.68 |
Table 3 Seasonal Zooplankton density (ind./m3) at Station 1 Al-Jewaber Bridge
The density of zooplankton ranged from 62.5ind/m3 in Autumn 2015 to 90.15ind/m3 in Winter 2016 Table 4. The average density was 73.3ind/m3. Crustaceans was the dominant groups constitute 93.1% of the total zooplankton and ranged between 50.6ind/m3 in Summer to 88.4 ind./m3 in Winter. Total Cladocerans comprised 50.1% then Copepods 49.2%, Rotifera 6.1% and Ostracods 4.0% of total zooplankton Figure 5. Cyclopoids was dominant of Copepoda that comprised 45.5% of total zooplankton and 92.6% of total Copepoda.
Zooplankton |
Autumn |
Winter |
Spring |
Summer |
Calanoida |
0 |
1 |
1.4 |
1.9 |
Cyclopoida |
38.2 |
12.5 |
53 |
30 |
Harpacticoida |
2 |
1 |
1 |
0.3 |
Nauplii larvae |
0 |
0.7 |
1.4 |
0 |
Total Copepoda |
40.2 |
15.2 |
57.1 |
32.2 |
Cladocera |
12.2 |
72.5 |
10.02 |
3.02 |
Insect larva |
0.1 |
0.7 |
0.8 |
1 |
Amphipoda |
2 |
0 |
5 |
0.5 |
Ostracoda |
0 |
0 |
1 |
10.9 |
Zoea of shrimp |
5 |
0 |
1 |
3 |
Total Crustacea |
59.5 |
88.4 |
74.9 |
50.6 |
Rotifera |
2 |
1 |
2.8 |
12 |
Fish larvae |
0 |
0.02 |
0 |
0 |
Polychaete larvae |
1 |
0.7 |
0.8 |
0 |
Gastropoda |
0 |
0.03 |
0 |
0 |
Total Zooplankton |
62.5 |
90.15 |
78.5 |
62.6 |
Table 4 Seasonal Zooplankton density (ind./m3) at Station 2 Hamayon Bridge
Station three representing of the Euphrates and TigrisConfluence. The density of zooplankton ranged from 71 ind./m3 in Autumn 2015 to 217.1ind/m3 in Summer 2016 Table 5. The average density was 142.4ind/m3. The Crustaceans was the dominant groups constitute 98.1% of the total zooplankton. Total Copepods comprised 44.9% then Cladocerans 33.0%, Cirripede larvae 17.7% and Insect larvae 1.6% of the total zooplankton Figure 6. Cyclopoids was dominant which comprised 41.6% of total zooplankton and 92.6% of total Copepoda.
Zooplankton |
Autumn |
Winter |
Spring |
Summer |
Calanoida |
0 |
0 |
0 |
1 |
Cyclopoida |
17 |
11 |
29 |
180 |
Harpacticoida |
1 |
4 |
3 |
5 |
Nauplii larvae |
1 |
2 |
1 |
1 |
Total Copepoda |
19 |
17 |
33 |
187 |
Cladocera |
44 |
88 |
30 |
26 |
Insect larvae |
3 |
2 |
4 |
0.1 |
Amphipoda |
0 |
0 |
0 |
1 |
Isopoda |
0 |
0 |
0.04 |
0 |
Ostracoda |
0 |
0 |
0.2 |
1 |
Cirripedia larvae |
1 |
0 |
100 |
0 |
Zoea of shrimp |
0 |
0 |
1 |
0.8 |
Zoea of crab |
1 |
0 |
0 |
0.1 |
Total Crustacea |
68 |
107 |
168.2 |
216 |
Rotifera |
3 |
3 |
2 |
1 |
Polychaete larvae |
0 |
0.8 |
0.6 |
0 |
Fish larvae |
0 |
0 |
0 |
0.1 |
Total Zooplankton |
71 |
110.8 |
170.8 |
217.1 |
Table 5 Seasonal Zooplankton density (ind./m3) at St. 3 (Shatt Al-Arab)
These results are compared with those reported by other authors in various parts of inland waters of Iraq, Mangalo and Akbar20 found that the density of zooplankton in Diyala River, further to the north of Basrah, was 861ind/m3 in February 1984 and only 0.4ind/m3 during November 1984. Whereas Mangalo and Akbar21, reported density of zooplankton 3843ind/m3 in January 1986 and 0.5ind/m3 in October 1985 in Diyala River and 3–172ind/m3 in July 1986 and March 1986, respectively in the Tigris River at Baghdad. Ajeel et al.4 reported the zooplankton density at St. 1 (Shatt Al-Arab at Al-Hartha north Basrah city) ranged between 110 ind/m3 in August 1996 to 1610ind/m3 in April 1997. While at St. 6 Shatt Al-Arab (near the city center at Al-Ashar) ranged between 125ind/m3 in January 1997 to 204ind/m3 in April 1997.
Seasonal changes varied in richness values in the study stations which recorded as the highest rate (1.66) during the summer and autumn, and lower rate (0.91) during winter and the average value was (1.46) at first station, and the highest rate (1.60) during spring and lower rate (1.33) during the winter and the average value was (1.46) at second station , while the highest rate was 1.56 during the spring and lower rate (0.85) during the winter and the average value was (1.27) in the third station Figure 7.
Varied diversity index values in the study stations was the highest value(1.41) during the spring and the lowest value (0.45) during the winter and the average value was (0.89) in the first station, while in the second station the highest value (1.61) recorded during the winter and the lowest value (0.99) during the spring and the average value was (1.01).Moreover, in the third station the highest value of diversity index was (1.14) during the spring and the lowest value (0.49) during the summer and the average value was (0.83) Figure 8.
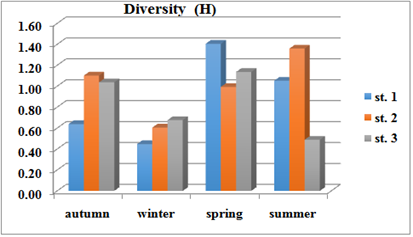
Figure 8 Seasonally variations of Diversity (H) at three stations during October 2015 to August 2016.
Figure 9 shows the annual average of Evenness index values, which recorded in the study stations. Evenness coefficient for the zooplankton reached highest value (0.68) during the spring and the lowest value (0.25) during the winter and the average value was (0.43) in the first station. Whereas in the second station the highest value (0.70) during summer and the lowest value (0.31) during winter and the average (0.51). While the highest value (0.58) during autumn and the lowest value (0.22) during summer and the average (0.43) in the third station.
Jaccard's similarity index was calculated for the zooplankton at three different stations; the highest similarity value was between stations (1) and (2) while the lowest value of the similarity between stations (1) and (3) Figure 10.
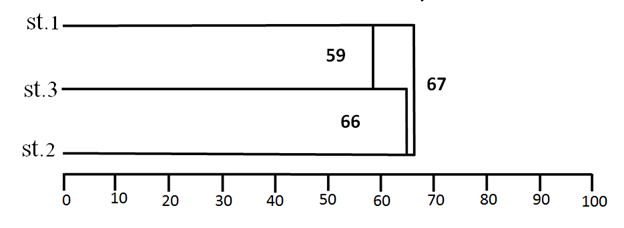
Figure 10 Cluster of the Similarity index (Jaccards) values of Group Zooplankton at three stations between October 2015 to August 2016 on a seasonally basis.
The results of the environmental evidence (Richness index (D), diversity index (H), Shannon Weaver and Evenness index (J) show low rate this evidence in the study stations has been attributed to pollution and environmental changes in this region and this is consistent with Al-Jizany22 and Ajeel and Abbas23 which reported the pollution due to reduce diversity index.
Correlation coefficient of environmental factors with zooplankton:
The correlation coefficients between the many physical and chemical properties included in the current study were calculated with the distribution of zooplankton. The results were as in Figure 11 shows the correlation between zooplankton and environmental factors, in the form, the Insect larvae group appears to correlate with chlorophyll-a significant and affected by other factors with little effect such as dissolved oxygen and temperatures. The effect of other physical factors is destitute. Gastropoda also affected by salinity, while the effect of Turbidity, water temperature and pH has little effect and chlorophyll-a and dissolved oxygen have no effect on this group. Significant positive relationships were found between Amphipoda, Rotifera, Copepoda, Fish larvae, Zoea of crab, Ostracoda, Zoea of shrimp and water temperature, whilethe effect of other environmental factors has had a weak.
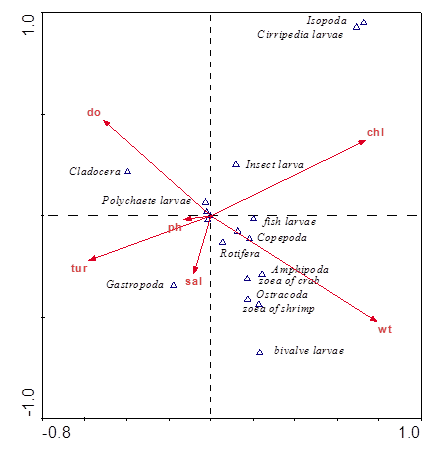
Figure 11 CCA analysis of the correlation coefficients between zooplankton and the environmental factors during the study period.
Table 6 shows a comparison of the density of zooplankton, Copepods, Cladocera and Cirripede larvae (ind/m3) in the current study compared with the previous studies in different regions and different stations at Marshes and Shatt Al-Arab.
|
Study Area |
Mish size |
Zooplankton |
Copepods |
Cladocera |
Cirripede |
References |
|
|
||||||
(mm) |
larvae |
||||||
1 |
Shatt Al-Arab |
0.09 |
110 - 2047 |
30 - 1322 |
0.3 - 229 |
0 - 187 |
4 |
2 |
Shatt Al-Arab |
0.09 |
70 - 27670 |
61-20067 |
4 - 10854 |
0 - 1802 |
24 |
3 |
Shatt Al-Arab |
0.12 |
6671 - 28064 |
4419 - 25821 |
0 - 24 |
269 - 1075 |
25 |
4 |
Al-Huwaiza Marsh |
0.12 |
61 - 3309 |
38 -3155 |
0.4 -72 |
0 -30 |
10 |
5 |
Al-Huwaiza Marsh |
0.12 |
21 - 9817 |
7 -1727 |
Sep-39 |
0 -261 |
26 |
6 |
Al-Izze river |
0.12 |
188 - 2714 |
168 - 2659 |
10 - 290 |
0 -5 |
10 |
7 |
Basrah Marshes |
0.12 |
52 - 2115 |
8 -1191 |
0.4 - 235 |
1.2 -1287 |
10 |
8 |
Al- Hammar Marsh |
0.12 |
197 - 8673 |
41 - 1361 |
61 - 6354 |
0 -2697 |
26 |
9 |
Al- Hammar Marsh |
0.09 |
717 - 1209879 |
79 - 40204 |
111 - 1095 |
79 -1185628 |
11 |
10 |
Central Marsh |
0.12 |
99 - 42655 |
48 - 20450 |
Jan-83 |
0 -47 |
26 |
11 |
Tigris River |
0.1 |
20 - 243 |
15 -142 |
1 - 211 |
0 -2 |
Present study |
12 |
Shatt Al-Arab River |
0.1 |
71 - 217 |
17 -187 |
26 -88 |
0 -100 |
Present study |
Table 6 Density of zooplankton, Copepods, Cladocera and Cirripede larvae (ind./m3) in different stations at Shatt Al-Arab River and Marshes
None.
None.
The author declares that there are no conflicts of interest.

©2019 Shaker, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.