Journal of
eISSN: 2373-6410


Research Article Special Issue 2 Migraine Profile in Sudan
1Faculty of medicine, University of Gezira, Sudan
2Taibah University, College of Medicine, Saudi Arabia
3Faculty of medicine, National Ribat University, Sudan
4Department of Radiology, National Ribat University, Sudan
5Faculty of medicine, Karrary University, Sudan
6Department of Medicine, Almaarefa Colleges, Saudi Arabia
7Radiology Unit, Royal Hospital, Sudan
Correspondence: Sara Ibrahim, Taibah University, College of Medicine, Saudi Arabia, Tel 966502813494
Received: October 10, 2017 | Published: November 27, 2017
Citation: Ibrahim SMH, Magzoub MSE, Nour AM, Nurein MS, Abdalla SF et al. (2017) Abnormal Circle of Willis among Migraineurs in Sudan. J Neurol Stroke 7(7): 00264. DOI: 10.15406/jnsk.2017.07.00264
Background: Several studies have shown a strong relationship between migraine and cerebral ischemia. The circle of Willis (CW) which is the main cerebral collateral pathway that allows flow redistribution in response to low perfusion is reported to be defective in migraine.
Objectives: This study aims to assess the structural characteristics of circle of willis (CW)in Sudanese migraineurs
Methods: This is a descriptive study including 46 migraineurs selected from a larger study (Migraine Profile in Sudan; a genetic, EEG and MRI study in 12 Sudanese pedigrees) and 100 apparently circle. Volunteers. All subjects underwent three-dimensional MRA of the cerebral arterial
Results: Defective Circle of Willis is observed in 5 % (n= 40) of migraineurs versus 68 % (n=68) of control. Fifty nine point six percent of migraineurs (n=28) and 53%(n=53) of control showed in complete configuration of CW. Hypoplastic vessels account for 46.8 % (n=22) in migraineurs especially in posterior circulation with 19 % (n=9) have hypoplastic both PcoA (P value 0.0001). While hypoplasia of either left or right but not both posterior communicating vessels was observed in 9% (n=9) of control Furthermore, frequent migraine attacks is significantly associated with hypoplastic arteries of the posterior circulation (P value 0.002).
Conclusions: Strong association observed between migraine and an abnormal configuration of the circle of Willis with hypoplasia of the both posterior communicating arteries significantly associated with frequent migraine attacks. This finding needs further confirmation as it will have an impact on the management and prognosis
Keywords: circle of willis, migraine with aura, migraine without aura
CW, circle of willis; MA, migraine with aura; MO, migraine without aura
Migraine has been connected to hypoperfusion and cerebral ischemia in several studies.1,2 Moreover, increased prevalence of silent white mater changes and even minute cerebral infarcts is reported in migraineurs.1 Cucchiara and Detre proposed that migraine can cause or result from cerebral ischemia.3 The basis of their assumption emerged from observations that catheter angiography can cause irritation of the endothelial and this may trigger cortical spreading depression (CSD) and eventually migraine attack.4 Hence, characteristic blood flow changes in CSD which include aninitial transient increased blood flow followed by a longer period of hypoperfusion might precipitate cerebral ischemia in some cases. The altered blood flow changes are thought to occur during the migraine aura,5 and demonstrated during headache in patients with migraine without aura as well.6
Cucchiara and Detre even went further to hypothesize a link between migraine and anomalies in the circle of Willis (CW). The presence of CW anomalies may contribute to the impaired regulation of cerebral blood flow in response to physiological activation known to occur in migraineurs.7 Altered cerebral blood flow (CBF) volume has been demonstrated in regions supplied by abnormal circle of Willis. Dysregulation of CBF may lead to relative ischemia to develop due to increased metabolic demand related to an elevated neuronal excitability, which may in turn trigger CSD, and eventually predispose individuals with migraine to ischemic lesions and stroke.3
The anterior cerebral circulation is connected to the posterior one by theposterior communicating artery forming a circular link called the Circle of Willis (Figure 1). The circle of Willis function as a collateral pathway that redistribute cerebral blood flow in cases of decreased perfusionin major arteries.8 The anatomy of the CW is exceedingly variable among populations, particularly in the anastomoses in the posterior circulation.9,10 These variations believed to occur during the fetal period due to developmental changes.3
ICA: Internal Carotid Artery; ACA: Anterior Cerebral Artery; MCA: Middle Cerebral Artery; PCA; Posterior Cerebral Artery; BA: Basilar Artery; VA: Vertebral Artery; Acomm: Anterior Communicating Artery; Pcomm: Posterior Communicating Artery; A1, A2, P1, P2: Branches of the Anterior and Posterior Cerebral Arteries.3
In 1998, Lovrencic-Huzjan et al.,11 found a significantly greater prevalence of vertebral artery hypoplasia in patients with migraine with aura compared with migraine without aura and controls.11 Another study found increased occurrence of fetal-type posterior cerebral artery (PCA) in patients with migraine with aura compared with matched controls.12 Both reported an association between incomplete posterior CW and migraine.13,14 The aim of this study is to assess the structural characteristics of the circle of Willis Sudanese migraineurs.
This is a descriptive study including 46 migraineurs and 100 apparently healthy control subjects recruited from medical staff and volunteers. The study took place at the radiology center, National Ribat Teaching Hospital in the period from January 2009- December 2010. Forty seven migraineurs from Phase I study who agreed to participate in this study together with 100 volunteers from the hospital staffs who have not suffered from, nor have reported family history of migraine. Both migraineurs and control ages ranged between 12-50 years old.
Experminetal Design and techniques
Magnetic Resonance Imaging (MRI): Cerebral imaging was performed on a 1.5 T MRI machine and included axial T1, T2, and FLAIR-weighted sequences.
Magnetic Resonance Arterial (MRA): MRA imaging of the Circle of Willis (CW) was performed using a 3 dimensional (3D) Time-of-Flight (TOF) sequence. Repetition time / echo time (TR/TE): 22 ms/3.5 ms; flip angle 15o; field of view: 220x220 mm; number of excitations: 1; slice orientation: transverse; slice thickness: 1 mm; number of slices: 64; scan percentage 100%, matrix reconstruction size: 512 x 512 resulting in a nominal voxel size (x, y, z) of 0.43x0.43x0.65 mm; total acquisition time: 5min 30sec.
The original MRA sequence for each patient was retrieved from an electronic database (DXMM®). Two investigators blinded to the clinical data rated the presence of CW segments on MRA. The statistical online software Vassarstat was used to analyze. The differences between sets of data from subjects with migraine and control were tested for significance by the unpaired t test. The effects on the results of various factors in the migraine group were tested with analysis of variance (ANOVA). The correlations between clinical variables and CW configuration were assessed by Pearson’s linear correlation test. Results were considered significant at P < 0.05.
Eighty five percent (n= 40) of migraineurs have defective CW (Figure 2) while only 15% (n=7) have normal configuration. Incomplete CW was observed in 59.5 % (n=28) migraineurs of whom, 25.5 % (n=12) have absent both PcoA, 12. 7% (n=6) have absent right PcoA, while absence of left PcoA and AcoA is seen in 8.5 % (n=4). Hypoplastic vessels account for 44.6 % (n=21), of these 17% (n=8) have hypoplastic both PcoA. Furthermore, frequent migraine is significantly associated with hypoplastic arteries of the posterior circulation (P value 0.002).
Recently, few studies reported that anomalies of the Circle of Willis (CW) are more prevalent in migraine patients than in the general population.13 According to Krabbe-Hartkamp et al.,15 CW anomalies are more frequent in the posterior circulation.15 Cerebral infarcts of migraineurs are even more commonly affect the posterior territory.1 Moreover, a number of studies have shown that an abnormal CW either with hypoplastic or missing segments affects regulation of regional cerebral blood flow16,17 which is also more evident in the posterior territory.18
In the present study, significant number of migraineurs showed defective CW (incomplete, hypoplastic and fetal type) especially of the posterior circulation (Figure 3). Our results are consistent with studies done by Bugnicourt et al.,13 who reported that an incomplete posterior CW was significantly more common in migraineurs than in the control group (49% vs 18%)13 and Cavestro et al.,19 who found a significant association between MO and variants and between MA and variants.19 A strong link is found between incomplete CW and MA by Cucchiara20 and colleagues in their recent work that also reported an accompanied altered cerebral blood flow.20 In contrast we found incomplete CW to be associated more with MA+MO followed by MO and least with MA (35,17. 8 and 7%) respectively. In disagreement to Ikeda et al we could not find any difference between control and migraineurs regarding the presence of fetal type CW.12
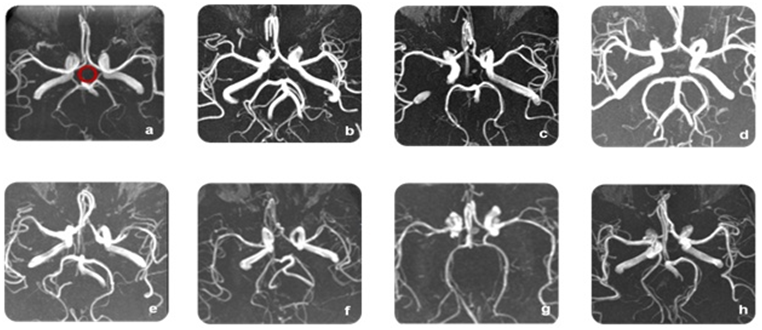
Figure 3 Structure of the circle of Willis in the study group: (a) Normal configuration, (b) Hypoplastic both posterior communicating arteries, (c) Absent Rt and hypoplastic Lt posterior communicating artery, (d) Absent Both posterior communicating arteries, (e) Absent Lt and hypoplastic Rt posterior communicating artery,(f)Absent Rt posterior communicating artery, (g) Hypoplastic Rt posterior communicating artery, (h) Fetal type.
In the present study, we found a significant association between migraine and hypoplastic posterior CW (Figure 3). Furthermore, severe migraine is associated with hypoplastic arteries of the posterior circulation, mainly the posterior communicating arteries (Table 1), a finding that was not reported before to our knowledge. In agreement to our results, regarding more involvement of the posterior circulation,Cavestro and colleagues also reported that hypoplastic basilar artery has been linked to migraine.19 Variations of CW among normal subjects have been reported9,10,21 with diverse figures for the complete circle; 90%.22,23 In Sudan, Alawad et al found complete CW in 72% while in the present study a complete CW observed in 32% of the control group versus 84% of migraineurs.24
|
Configuration of the Circle of Willis |
Migraineurs |
Control n=100 |
Value |
|
Defective |
n=40 |
n=68 |
0.04 |
|
Hypoplastic |
n=21 |
n=9 |
0.0001 |
|
Incomplete |
n=28 |
n=53 |
0.28 |
|
Fetal type |
n= 3 |
n=6 |
0.59 |
Table 1 Showed association defective; hypoplastic Incomplete circle of Willis and fetal type between migraineurs and control
Nitrates trigger while nitric oxide synthase inhibition abort migraine attacks.25˗27 Upon exposure to induced ischemia, endothelial nitric oxide synthase (eNOS) knockout mice develop larger infarcts attributable partly to defective vasodilatation and decreased collateral blood flow. Inversely, gene-deletion of neuronal nitric oxide synthase (nNOS) confers neuroprotection.28 When dissected, these mice were found to have defective circle of Willis.29 Hence, an association between nitric oxide and alterations of the circle of Willis is expected. An assumption that needs verification.
Strong association is observed between migraine and an abnormal configuration of the circle of Willis with hypoplasia of both posterior communicating arteries significantly associated with more frequent attacks of migraine. This finding needs further confirmation as it will have an impact on the management and prognosis.
We gratefully acknowledge the assistance of the Sudanese ministry of higher education, for partially funding this research.
None.
None.

©2017 Ibrahim, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.