eISSN: 2377-4304


Research Article Volume 13 Issue 3
Cardiovascular Pathophysiology Research Unit (CPRU), Physiology Department, Faculty of Basic Medical Sciences, College of Health Sciences, Usmanu Danfodiyo University, Nigeria
Correspondence: Adamu Jibril Bamaiyi, Cardiovascular Pathophysiology Research Unit (CPRU), Physiology Department, Faculty of Basic Medical Sciences, College of Health Sciences, Usmanu Danfodiyo University, Sokoto, Nigeria, Tel +2348030925695
Received: May 24, 2022 | Published: June 22, 2022
Citation: DOI: 10.15406/ogij.2022.13.00647
Third trimester of pregnancy is characterised by profound changes in the haemodynamic system, predicated by the climax of the pregnancy hormones profiles. The blood pressure (BP) is lower and the haemoglobin levels are lower, necessitating an increased circulatory work to maintain tissue perfusion of the new maternal-fetal placental bed and for the expectant blood loss during childbirth. However, the mechanisms by which this is achieved is poorly understood. The present study aimed to assess the mechanisms by which the mother’s systems adjust to meet its haemodynamic gaps at the third trimester of a normal pregnancy. One hundred and fifty normal third trimester pregnant women undergoing antenatal care at the State Specialist Hospitalist Sokoto were assessed for blood pressure, haemoglobin concentration and anthropometries and the results compared with those of a group comprising 115 non-pregnant control women. While the pregnant group had lower BP parameters, it has significantly higher PR (P<0.0001). The pregnant group also demonstrated higher rate pressure product (RPP) (10,196±1,292bpm.mmHg v 9,429±1,469bpm.mmHg, P=0.0000). However, the non-pregnant group showed significantly higher heart rate reserve (HRR) (108.0±13.0bpm.mmHg/year), compared to the pregnant group (96.0±12bpm.mmHg/year, P=0.0000). Although, after adjusting for age, the pregnant group maintained a significantly higher RPP (397±120bpm.mmHg/year v 358±139bpm.mmHg/year, P =0.0156), the non-pregnant group after adjustment for age failed to maintain a higher HRR, instead the pregnant group showed (4.0±1.0bpm/year v 4.0±2.0bpm/year, P=0.0328). In conclusion, the pregnant woman can adjust haemodynamically by mechanisms of RPP and HRR independent of age, to cope with the stress and requirements of pregnancy. This, in addition to supplementation of haematenics, appropriate treatment of common infections and better nourishment of the pregnant mother, to help improve the anemia during pregnancy.
Keywords: third trimester pregnancy, anaemia, blood pressure, rate pressure product, heart rate reserve
Anaemia in pregnancy, considered as physiological, is a well-known phenomenon.1,2 It has been explained to result from volume expansion necessitated by the physiological adjustments predicated by hormonal changes that comes with normal pregnancies.3,4 However, the extent of the anaemia during pregnancy depends on the population studied, as well as the social and economic status of the population studied.1,5 The prevalence of anemia in pregnancy has been reported to vary between 14% in high income countries in Europe, up to 61% in some West African countries.5–7 Furthermore, the haemoglobin levels in pregnancy tends to decline further as the pregnancy advances to term.5,6,8 In this regard, even pregnant women taking routine haematenic supplements have been found to have the anemic trend due to varied and disproportionate requirements and hormonal changes.9,10 Indeed, iron supplementation as traditionally practiced during antenatal services, essentially corrects iron deficiency form of anaemia and may be appropriate only for the malnourished category of pregnant mothers.9 Therefore, there are far many causes of anemia in pregnancy that iron supplements alone cannot address.9 However, the last two decades have witnessed improvement in the levels of haemoglobin in women, and of course children.
Although studies (commonly among non-pregnant women), have shown association between anaemia and morbidity and mortality,7,11 but this may not be exactly the same of pregnancy state.6 And although, a recent review paper.9 attributed low birth weight and hypertensive disease of pregnancy to anaemia, a multicentre and multiethnic study6 showed no significant relationship between anemia in pregnancy and adverse birth outcome.
Anaemia in pregnancy is associated with haemodynamic adjustments, in order to make-up for oxygen and nutrient requirements of both the mother and fetus.12,13 Of course, the physiology of the human body requires that the anemic pregnancy state will invoke compensatory mechanisms to cope with oxygen and nutrient demands of both the mother and the fetus, but this has not been clearly explained in the literature, as yet. Further, most of the haemodynamics changes during pregnancy climax at the third trimester, owing to accentuation of the hormonal changes,14 but the mechanisms by which the mother’s systems adjust to meet its haemodynamic gaps at this stage of pregnancy has not been made plain in the literatures.
Importantly, the components of haemodynamics especially blood pressures (BP) are not known to change significantly between beginning and the last part of pregnancy, but the HR does increases.15 However, the extent to which these variables adjust with anaemia of pregnancy and those mechanisms that could explain them to maintain tissues oxygenation and nutrition is not well-understood. In this regard, how much oxygen does the heart of a pregnant woman need and what further adjustment could the HR go to cope with the intravascular expansion in the face of decreased blood oxygen carrying capacity, are important issues to address. Again, the mechanisms by which these are achieved during pregnancy has not been properly explained in earlier studies. Consequently, in the present study we evaluated the anthropometries of both groups, estimated the haemoglobins (Hb) and packed cell volumes (PCV) of the groups, compared the systolic pressure (SP), diastolic pressure (DP), Mean arterial pressures (MAP) and heart rates (HR) of the 2 groups. We further evaluated the rate pressure product (RPP), a well-verified index for myocardial oxygen consumption and we also compared between the groups, the heart rate reserve (HRR) before and after adjustment for age.
Ethical issues and sampling
The ethical permission for this study was obtained from the Sokoto State Specialist Hospital Committee for Human Research (Ref: SHS/SUB/133/VOL I). Every participant has signed an informed consent form before enrolling in the study. And the rules and regulations guiding human research as declared at Helsinki, Finland by the World Medical Association was adhered to.
The study was a longitudinal descriptive one where each participant had 3 contact periods with the researchers within one month, at 2 weeks interval. One hundred and fifty consecutive and consenting third trimester pregnant women from Sokoto metropolis, attending antenatal care at the state Specialist Hospital Sokoto were recruited for the study. And they were compared with 115 non-pregnant matched control women sourced from the same premises of the hospital.
Inclusion criteria
Third trimester pregnant women, not less than 18 years of age, carrying singleton pregnancy, not suffering from any chronic illnesses and does not smoke cigarette or consume alcoholic beverages were recruited for this study.
Exclusion criteria
Not included in the study were participant younger than 18 years, not of black African descent, those not from Sokoto metropolis and those not willing to stay in the study to completions were not included. Also, non-pregnant participant within the puerperal period were not enrolled.
Measurements
Weight: Weight of each participant was taken 3 times, two weeks apart using a portable weight scale (Camry, China, ISO 9001: 2008 certified by SGS, model: BR 9012) following standard procedures as earlier described.8
Height
This was taken once during the first contact, and barefooted in accordance with best practices using the meter rule described.8
BMI
This was derived from the body weight and height using the relationship
BMI = Weight (kg)/Height squared (m2)
MUAC
This was taken thrice, 2 weeks apart for each participant using an inelastic but flexible measuring tape (China, Butterfly). The details of the procedure have been described previously.8
Blood pressure
This was taken at each of the 3 contact periods, in sitting position using an Omron (Japanese M2 basic) digital upper arm blood pressure monitor, after 5 minutes of rest. The machine has 2 adaptable cuffs, suitable for the variations in upper arm circumferences. The other procedures followed standard protocols, and has been previously documented.16 The average of 3 readings were used as the systolic (SBP), diastolic (DBP) blood pressures, as well as the pulse rate (PR).
The pulse pressure (PP): Was derived from the relationship: PP=SP–DP (mmHg)
Rate pressure product (RPP): A well-known index for estimation myocardial oxygen demand. The RPP is obtained from the relationship RPP =PR x SBP (bpm.mmHg).
Heart rate reserve (HRR): A measure of how much further an individual HR could be increased without any untoward effect. Consequently, HRR was obtained from the popular Fox’s age-based relationship: HRR = Predicted maximum
HR (HRmax) - Resting HR (HRrest) =220 – Age (years) – HRrest17
Haemoglobin (Hb) and Haematocrit (Hct)
Blood was withdrawn following standard procedures as previously described [8], and the blood transferred into a 5mls bottle anticoagulated with EDTA-2K. Automation method was used to analyze the blood using Full Automatic Blood Cell Counter, PCE – 210E, ver.5. 10 (Erma, Tokyo). Thereafter, the results were retrieved from the printout and recorded as Haemoglobin (Hb) and Haematocrit (Hct).
Statistics
Data base storage and analysis was carried using Microsoft excel system (Windows 10 pro, 2017 Microsoft Corporation). Exploratory data analysis was done to identify and address missed or incorrect entries. Values were expressed as mean +/- standard deviations for continuous variables or as percentage where they are presented as proportions. Tables and charts were used to present some parts of the data. Difference in means between groups were decided with t-test and the P-values were pegged at <0.05.
In this study, haemodynamic indexes were assessed among 150 third trimester pregnant women undergoing antenatal care at the State Specialist Hospitalist Sokoto and compared the results with those of a group comprising 115 non-pregnant control women. Both groups were age-matched; mean age of the pregnant group was 27.2±6.2 years and the non-pregnant control was 28.4±7.2 (P =0.1452).
Most of the participants in the study are Hausa/Fulani and Muslim. See table 1. Other demographic characteristics of the participants are also shown in Table 1.
|
|
Pregnant group (n=150) |
Non-pregnant group (n=115) |
Tribes |
Hausa/Fulani |
104 (69.1%) |
52 (45.0%) |
Yoruba |
22 (14.7%) |
22 (19.5%) |
|
Igbo |
8 (5.3%) |
23 (20.0%) |
|
Others |
16 (10.7%) |
18 (15.5%) |
|
Religions |
Islam |
106 (70.7%) |
76 (66.1%) |
Christianity |
44 (29.3%) |
39 (33.9%) |
|
Educational level |
Arabic |
51 (33.7%) |
- |
|
Primary |
4o (27.0%) |
13 (11.5%) |
Secondary |
32 (21.4%) |
17 (15.0%) |
|
Post-secondary |
27 (17.9%) |
85 (73.5%) |
|
Employment status |
Unemployed |
105 (70.0%) |
- |
Students |
9 (6.0%) |
28 (24.5%) |
|
Business |
34 (22.7%) |
12 (10.3%) |
|
|
Civil servant |
2 (1.3%) |
75 (65.2%) |
Table 1 Study participants characteristics
Although, both groups showed no significant difference in height, (1.6±0.0 vs 1.6±0.0, P=0.0705) and whole body weight (68.5±1.1 vs 68.3±2.3, P=0.3627), there were significant differences in the BMI in favour of the pregnant group (26.8±0.4 vs 26.2±0.9, P=0.0000), and the mid upper arm circumference (MUAC) was greater in the non-pregnant group (27.7±4.2 vs 30.0±0.5, P =0.0000).
Both the Haemoglobin (Hb) levels and percentage Packed Cell Volume (PCV) were significantly lower among the pregnant group, compared to the non-pregnant control group. Indeed, these values steadily dropped in the pregnant group, during every of the 3-contact period of the study, but the values remain essentially constant in the non-pregnant group. See Figure 1a and Figure 1b.
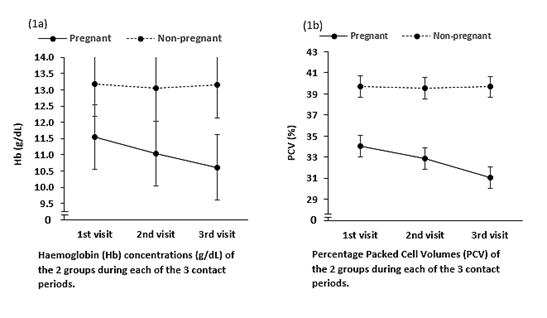
Figure 1 Values steadily dropped in the pregnant group, during every of the 3-contact period of the study, but the values remain essentially constant in the non-pregnant group.
Similarly, all the haemodynamics indexes except, pulse rate (PR) was significantly lower in the pregnant group compared to the non-pregnant group. In this regard, the PR in the pregnant group was significantly higher than the pregnant group (97±10 bpm v 83±10 bpm, P=0.0000) (Figure 2).
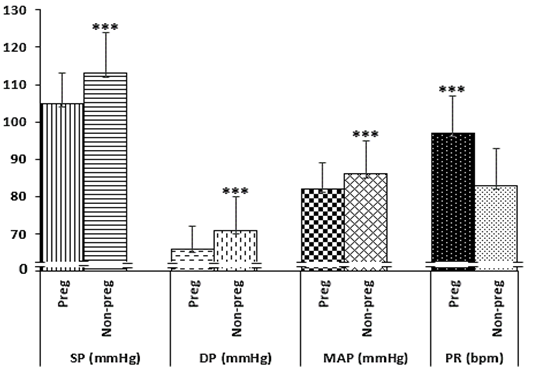
Figure 2 Comparison of the blood pressure indexes of the 2 groups.
NB: Systolic pressure (SP), Diastolic pressure (DP), Mean arterial pressure(MAP), Pulse rate (PR). P<0.000 (***)
Furthermore, when the rate pressure product (RPP), an index of myocardial oxygen demand were compared between the 2 groups, the pregnant group showed significantly higher values compared to the non-pregnant group (10,196±1,292bpm.mmHg v 9,429±1,469 bpm.mmHg, P=0.0000). See Figure 3a.
However, comparing the Heart Rate Reserve (HRR) between the groups, the non-pregnant groups showed a higher reserve 108.0±13.0bpm, compared to the pregnant group with reserve of 96.0±12 bpm (P=0.0000). See Figure 3b.
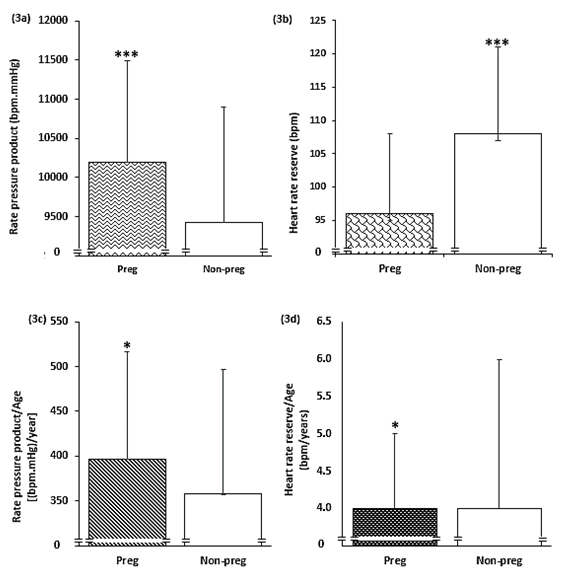
Figure 3 Comparison between the pregnant and non-pregnant groups (3a) Rate pressure product, (3b) Heart rate, (3c) Rate pressure product adjusted for age and (3d) Heart rate reserve adjusted for age.
NB: Pregnant group (Preg), Non-pregnant group (Non-preg), P<0.05 (*), P<0.005 (**), P<0.001(***)
Although the pregnant group maintained a significantly higher RPP after adjustment for age (397±120bpm. mmHg/year v 358±139bpm.mmHg/year, P=0.0156), see Figure 3c, the non-pregnant group failed to have a higher HRR after adjustment for age, instead the pregnant group maintained higher HRR as shown in Figure 3d (4.0±1.0 bpm/year v 4.0±2.0bpm/year, P=0.0328).
The following have been reported in the present study; that in a Hausa/Fulani and Muslim dominated sample of third trimester pregnant women, with most of them on the low socio-economic scale, the Hb and Hct declines as the pregnancy advances to term, compared to their age- matched non-pregnant female counterparts. That although the BP indexes are significantly lower in the pregnant group, the HR was higher in the pregnant group. Also, that RPP is significantly higher in the pregnant group, but the HRR is lower in them. However, adjusting both RPP and HRR for age, the non-pregnant group fails to maintain the dominance, instead the pregnant group did.
Haemoglobin/Haematocrit
There was a steady decline in both Hb and Hct during the visit periods. This may not be unconnected with the heamodilution obtainable during normal pregnancy.18 The hormonal change of pregnancy creates an unfilled vascular spaced owing to systemic vasodilation and increased capacitance of the vessels that needed to be filled, hence the volume expansion.8,19 In this regard, there is pregnancy-induced elevation in plasma renin with a relative drop in the atrial natriuretic peptide (ANP) levels.19 Moreover, the predominantly lower socio-economic status of the pregnant group in the present study might have contributed to the level of anaemia due to poor nutrition. This may be corroborating the findings of Akinbami and colleagues20 in Lagos, and Oyelese and collleagues21 in Ogun state, both in South-west Nigeria where low socio-economic status was identified as an important contributor to anaemia in pregnancy. Furthermore, anaemia in pregnancy in low-income countries as Nigeria are usually characterized by infections such as malaria, which is endemic in Nigeria.11,20 Furthermore, increasing age, in addition to maternal infection and low socio-economic status were the leading explanations for anemia in pregnancy.20,21 Similarly, in a related study at faraway Morogoro municipality of Tanzania, by Msolla and kinabo,22 it was reported that even though the pregnant women enrolled had good awareness about anemia of pregnancy, anemia was rife among them and were attributed to lack of food security, poverty, infection and iron deficiency. And it is in tandem with the findings of Finkelstein et al.,23 among pregnant women in Indian, where aside from the high prevalence of anemia among the subjects, there is prominent associatio between the level of anemia and pregnancy outcome. However, in the Indian study by Finkelstein and collegues,23 anemia in pregnancy was more severe in the first trimester than in other semesters. The disparate result of the Indian study and the earlier ones, like ours, may be explained by the fact that we didn’t compare the haemoglobin levels of the different troimesters, instead we focused only on the third trimester.
Haemodynamics
Assessing the BP indexes, the pregnant group showed lower systolic, diastolic and mean arterial BPs, compared to the non-pregnant control group (P<0.000). This is in consonance with a earlier similar study24 where BP was reported to drop because of the predominantly systemic vasodilation that occurs in pregnancy, but not other studies,25–27 where no significant changes in BP were observed and there may be an increase in the BP parameters.15,28 In this regard, the study in Tokyo conducted by Jwa and colleagues,29 stated the exception to BP fall in mid-pregnancy is only among the category of pregnant women prone to developing pregnancy induced hypertension (PIH). Furthermore, Silva et al.30 did not find a fall in either systolic BP or diastolic BP at mid-pregnancy among the subjects enrolled in their study, except among the participants with level of education. Indeed, BP is widely reported to decrease in the first 24 weeks of pregnancy, then rises subtly again after that time till childbirth.31 Nevertheless, the values are still less than what obtains in the age matched non-pregnant women, because of the predominant systemic vasodilation of pregnancy.19 In this regard, higher BP in pregnancy may be associated with untoward outcome.32 The discrepancies in the BP results in these earlier studies, especially compared to ours, may not be unconnected to the failure of the earlier studies to compare their results with non-pregnant matched control women. This is a precaution the present study took.
However, the PR among the pregnant group was significantly higher (p=0.000). This is similar to the findings of Ishikuro and colleagues,33 where they reported increase in pulse rate (PR) steadily during normal pregnancy till childbirth and it may be in compensation for the lower BPs obtained during pregnancy,24 in the face of higher requirements for an increased circulation and perfusion of the new maternal-fetal placental bed and for the expectant blood loss during childbirth.12,13 In this regard, cardiac output increases as pregnancy advances, entailing increase d HR and work of heart, with the former predominating. This observation is consistent with the findings of34 when the HRs were compared between pregnant and non-pregnant mice, but contrary to HR in pregnancy reported by Loerup, Pullon35 where there was no such significant difference in PR as reported in earlier studies.
The data in the present study also showed a significantly higher myocardial oxygen consumption in the pregnant group evidenced by the higher RPP. Of course, the anaemic state with substantial volume expansion in pregnancy translates to requirement for more work to be done by the myocardium to meet tissues perfusion. This comes with associated oxygen demand by the cardiac tissues36 which the control group does not require. Indeed, the present study is different from some earlier studies,37–39 to the extent that they were not conducted in normal pregnant women, instead the conclusions were drawn among heart failure patients.
However, non-pregnant group has higher HRR, within which to adjust further. The HRR provides an information on the limit above which the HR could not be increased without adverse effect on the cardiovascular system.40 On this, the pregnant group has already attained higher HR, in order to cope with the haemodynamic needs, limiting further increases.
Importantly, the HRR is profoundly limited by age. Therefore, after adjustment for age the non-pregnant group no longer have higher HRR, instead the pregnant group did. Further, the pregnant group continued to have significantly higher RPP even after adjustment for age. This may explain why the findings of the systematic review and meta-analysis by Beetham and colleagues,41 that vigorous aerobic exercise (which expectedly is associated with significant heart rate) carried out during the third trimester of pregnancy has no adverse effects on the pregnancy outcome. Indeed, moderate regular exercise in third trimester of pregnancy influences control of diabetes, hypertension, weight gain and incidence of caesarian section, hence the physiological tendency of a normal pregnancy to achieve
Limitations of the study
Pulse rate was used for HR, which may not be appropriate if there exist some cardiovascular disease conditions that will cause pulse deficit. However, our participants do not have any apparent cardiovascular conditions as such. The iron levels could not be assessed to explain the type of anaemia the participants have to explain the type of anaemia our participants are. However, considering their demography, the most likely type of anemia, which is essentially the commonest type in low-income countries is iron deficiency anaemia, arising from poor nutrition. Also, infectious, or other haemolytic causes of anaemia were not screened in the participants, but we ensured that the participants were not sickle anaemia patients and they were not having any clinical symptoms of infections evidenced by normal body temperature, as of the time of the contacts.
Although, the third trimester pregnant women in our setting have lower BP parameters compared with non-pregnant women and have lower haemoglobin concentrations, they were able to adjust haemodynamically by mechanisms of RPP and HRR independent of age, to cope with the stress of pregnancy. However, haematenics supplementation, appropriate treatment of common infections and better nourishment of the pregnant mother may help improve the anemia in the pregnant woman in our setting and may alleviate the haemodynamic stress that they undergo as a result of deficiencies of the above factors.
The management and staff, especially of the Obstetrics and Gynecology Department of the State Specialist Hospital, Sokoto, are hereby appreciated. I am sincerely grateful to the participants in this study. All the participants especially the already stressed pregnant ones were amazing. I am also indebted to the staffers of the Physiology Department, Usmanu Danfodiyo University, Sokoto, especially Mallam Abdullahi Tanko of the Department, who assisted importantly with crowd control and some local running during the study activities.
Author’s contribution
The author conceptualized the study, planned it, was in the frontline in the data collection, he analysed the data and chart a direction for the result and wrote up the manuscript. He also takes responsibilities for the content of the manuscript.
Data availability
The data for the present study may be made available, upon request from the author, provided the use to which the data is intended is confirmed genuine and justified.
Ethical approval
The present study, only commenced after gaining the written approval of the Sokoto State Specialist Hospital Committee for Human Research (Ref: SHS/SUB/133/VOL I). Every participant also signed an informed consent form before enrolling in the study. And the rules and regulations guiding human research, enshrined in the declaration of Helsinki, Finland by the World Medical Association were adhered to.
None.
The author declares that there is no conflict of interest.

©2022 . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.