eISSN: 2377-4304


Research Article Volume 13 Issue 4
1Medical assistant of Obstetrics and Gynaecology. Complexo Hospitalario Universitario de Ourense, Spain
2Head of the Gynaecology Department. Department of Obstetrics and Gynaecology. Complexo Hospitalario Universitario de Ourense, Spain
Correspondence: Santa María-Ortiz Johana Karin, Medical assistant of Obstetrics and Gynaecology. Complexo Hospitalario Universitario de Ourense, Calle Ramón Puga Noguerol, 54, 32005, Ourense, Spain, Tel +34625521289
Received: June 25, 2022 | Published: July 11, 2022
Citation: DOI: 10.15406/ogij.2022.13.00649
History: Bacterial vaginosis has been seen to have a negative impact on the reproductive outcomes of in vitro fertilization (IVF).
Aim: To determine its impact on the rates of biochemical pregnancy, clinical pregnancy, early spontaneous miscarriage and live newborns.
Data source: Bibliographic search according to PRISMA guidelines in the MEDLINE, EMBASE, CINAHL and Cochrane Library databases.
Eligibility criteria for the studies: The process for identifying and selecting studies is shown in the PRISMA flowchart. Evidence was evaluated according to the GRADE method.
Subjects and interventions: Infertile women with IVF. Diagnosis of bacterial vaginosis according to Nugent or qPCR criteria.
Evaluation of studies and summary methods: Forest plot, sensitivity analysis, funnel plots and evaluation of evidence according to GRADE.
Results: A total of seven studies were included. We detected an overall statistically significant association with the rate of biochemical pregnancy (OR 0.55; 95%CI: 0.36-0.85; P=0.004) and rate of clinical pregnancy 0.43; 95%CI: 0.22-0.87; P=0.018). This was not the case for early spontaneous miscarriage (OR 1.13; 95%CI: 0.46-2.82; P=0.78) and rate of live newborns (OR 1.63; 95%CI: 0.61- 4.32; P=0.33).
Limitations: Observational studies with a small sample and significant heterogeneity between them.
Conclusions: Bacterial vaginosis appears to have some impact on the rate of clinical and biochemical pregnancy achieved with IVF.
Keywords: abnormal vaginal flora, bacterial vaginosis, biochemical pregnancy, clinical pregnancy, early spontaneous miscarriage, infertility, in vitro fertilization, live newborns
Kirmani, 19881 discovered that lactobacilli are the dominant microflora in the vaginal ecosystem. They play an essential role in maintaining the natural balance of the vaginal flora and protecting women from genital infections. They produce lactic acid, hydrogen peroxide, bacteriocins, glycogen and glycerol that help defend against pathogens and prevent their predominance.2–6
There are various situations in which the vaginal flora’s balance is modified. Bacterial vaginosis (BV) is the most common genital disorder in women of childbearing age.7 This disorder is marked by microbial dysbiosis that modifies the normal acid environment, dominated by Lactobacillus spp., to a more heterogeneous setting with a higher number of strict and facultative anaerobic bacteria such as Gardnerella vaginalis, Mycoplasma hominis, Atopobium vaginae and Mobiluncus curtisii.7–10 Several approaches for diagnosis have been used. Most common are the clinical criteria of Amsel, 198311 and the Nugent score, 1991.12 The latter is based on the absence of lactobacillus morphotypes and existence of bacterial morphotypes associated with typical BV in a Gram-stained smear. In the last few years molecular techniques have arisen as promising tools to characterize the vaginal flora (qPCR). They dichotomize the vaginal flora into normal and abnormal and they enable subclassifying BV based on a quantitative evaluation of pathognomonic bacterial species for BV.13–16
It has been reported that the prevalence of BV in the infertile population is 19%.17 This may be asymptomatic in up to 50% of cases.17,18 An abnormal vaginal microbiota has been associated with a poor reproductive outcome in patients who undergo in vitro fertilization (IVF).19–25 However, the existence and predominance of lactobacilli has been associated with a higher rate of pregnancy.26,27 It has been reported that vaginal bacteria go up to the endometrium and generate bacterial contamination during transfer of embryos. There is increasingly more evidence that bacterial contamination of the uterine cavity, after transcervical embryo transfer through the catheter tip, may lead to a reduction in endometrial receptiveness, failed implantation and, therefore, reduced rates of pregnancy.28–32
To date, few studies have been performed on women who have undergone IVF. There are contradictory results in regard to the relationship between the abnormal vaginal flora and reproductive outcomes. Moore et al.,26 detected better birth rates for IVF in those women with a higher prevalence of Lactobacilus spp. in the vaginal flora. Haar et al.,19 suggested a significant negative impact of BV-like vaginal flora on the rate of clinical pregnancy. Mangot-Bertrand et al.,33 also detected a relationship between BV and reduction in the rate of embryo implantation, pregnancy, early spontaneous miscarriage and premature birth rate. However, this was not statistically significant. The heterogeneity between the populations of women studied, different technical approaches and different sampling times, hinder direct comparisons between the studies and prevent drawing robust conclusions.19,22,33–35 Therefore, the effect of the composition of the vaginal flora on reproductive outcomes of IVF, remains controversial.
Literature search methodology
The systematic search meticulously followed the guidelines of the PRISMA checklist36 and the 6-step process established by Part E. Systematic Review Literature Search Methodology.37 Moreover, a systematic literature search was performed in MEDLINE, EMBASE, CINAHL and the Cochrane Library, using MeSH terms and the inclusion and exclusion criteria set out in Tables 1–3. Only studies published in English were used. Two independent reviewers selected the studies that potentially complied with the inclusion criteria. They were initially selected by the title and subsequently according to the abstracts. Disagreements were resolved by means of a discussion between the two review authors.
The methodological quality of the studies selected was evaluated by means of applying the MOOSE guidelines for non-random studies.38 The research question was devised by means of the PICO strategy (Table 1).39 The main aim was to determine the effect of BV on reproductive outcomes of IVF by analysing the rates of biochemical and clinical pregnancy, early spontaneous miscarriage and live newborns. The secondary aim was to evaluate whether the impact of BV on the above events remains stable or is modified according to the diagnostic criteria used.
Component |
Specification |
Poblation |
Infertile women, for any reason, with in vitro fertilization. |
Indicator |
Bacterial vaginosis or abnormal vaginal microflora. |
Control |
Normal vaginal microbiota. |
Outcomes |
Biochemical pregnancy rate, clinical pregnancy, early spontaneous |
Table 1 PICO eligibility criteria
The study identification and selection process is shown in the PRISMA flowchart (Figure 1). Quality of evidence was evaluated by means of the methods reported in the GRADE manual40 (Appendix 1). The inclusion and exclusion criteria used are reflected in Table 2.

Figure 1 Diagram of selection of PRISMA studies for the meta-analysis of the relationship between BV and IVF results.
Inclusion criteria |
Exclusion criteria |
1. Articles in English, published peer-reviewed journals. |
1. Articles in a language other than English. |
2. Women of reproductive age. |
2. Full text not available. |
3. Studies focused only on IVF or ART. |
3. Animal studies. |
4. Subfertility or female infertility. |
4. Articles that analyze only the male microbiota. |
5. Human studies. |
5. Studies that analyzed BV with Amsel criteria or other criteria other than Nugent and qPCR. |
6. Studies that analyzed BV with Nugent criteria or qPCR |
|
Table 2 Inclusion and exclusion criteria
MESH terms |
|
Disease |
Infertility, subfertility. |
Outcome measures |
Fertilization in vitro, pregnancy, conception, IVF success, IVF outcome, fertilization, biochemical o chemical pregnancy, clinical pregnancy, early miscarriage, spontaneous miscarriage, early spontaneous abortion, live newborn, live birth. |
Methodological terms |
Prospective studies, prognosis. |
Patient characteristics |
Female, human, reproductive age, fertile, infertile, subfertile. |
Prognostic factors |
Bacterial vaginosis, vaginal microbiome, vaginal microbiota, vaginal microflora, cervical o cervix microbiome, cervical o cervix microbiome, bacterial dysbiosis. |
[microbiome OR microbiota OR ´omics’] AND [infertility OR subfertility] |
|
[microbiome OR microbiota OR ´omics’] AND [infertility OR subfertility] |
|
AND [pregnancy OR miscarriage] |
|
[microbiome OR microbiota OR ´omics’] AND [IVF OR Fertilization in vitro] |
|
[microbiome OR microbiota OR ´omics’] AND [IVF OR Fertilization in vitro] AND [pregnancy OR miscarriage] |
|
[microbiome OR microbiota OR ´omics’] AND [assisted reproductive technologies OR assisted reproduction OR ART] |
|
[microbiome OR microbiota OR ´omics’] AND [vaginal OR vaginal] |
|
|
[microbiome OR microbiota OR ´omics’] AND [cervix OR cervical] |
Table 3 MESH terms
Bacterial vaginosis evidence profile and IVF results |
|||||||||
Quality assessment |
Summary of results |
||||||||
Nº of studies |
Design |
Risk of bias |
Inconsistency |
Imprecision |
Publication bias |
No. of patients |
Magnitude of effect (95% CI) |
Quality |
Importance |
Outcome 1: Biochemical pregnancy |
|||||||||
6 |
Observational studies |
Likely |
Sí p=0,07; I2 =48% |
Likely |
Likely |
1185 |
OR 0,67: 0,39-1,15 |
Very low |
Important |
Outcome 2: Clinical Pregnancy |
|||||||||
6 |
Observational studies |
Likely |
Sí p<0,01; I2 =64% |
Likely |
Likely |
1474 |
OR 0,54: 0,28 -1,03 |
Very low |
Important |
Outcome 3: Early miscarriage |
|||||||||
5 |
Observational studies |
Likely |
No p=0,75; I2 =0% |
Likely |
Unlikely |
406 |
OR 1,38: 0,66 -2,88 |
Very low |
Important |
Outcome 4: Live Newborns |
|||||||||
4 |
Observational studies |
Likely |
No p=0,49; I2 =0% |
Likely |
Likely |
376 |
OR 1,08: 0,50-2,33 |
Very low |
Important |
Appendix 1 Quality of evidence according to GRADE
No restrictions to the publication date or type of design were applied. Papers were selected for subsequent evaluation when at least one of the following terms were found in the title or abstract: composition of vaginal flora, vaginal flora, bacterial vaginosis or abnormal vaginal flora associated with IVF results. Study variables were rates of biochemical pregnancy, clinical pregnancy, early spontaneous miscarriage and live newborns. Studies were considered eligible if BV was diagnosed by means of standardized Nugent criteria12 or by means of qPCR stratification.13 Given that the Amsel criteria11 have been thought to be more subjective, studies performed with these criteria were excluded. Data extraction included the following study characteristics: author and year of publication, data source, sample size, methodology, study aims and results.40
Statistical analysis
A meta-analysis was performed with the help of the statistical software “R- project for statistical computing 3.5.2”, for each study variable. This was represented in each case by means of forest plot. Heterogeneity was reported in the form of between study variance (tau2) and standard error for each estimate. A value P<0.05 in the heterogeneity test reflected statistically significant heterogeneity. Inconsistency (I2) represented the percentage variation observed between studies. A value higher than 0% indicated increasing heterogeneity. When tests revealed between study heterogeneity, the random effects model was used to estimate the weighted average effect.41 The main summary measures were the odds ratio (OR) and 95% confidence interval. Finally, the funnel plot was used to investigate probable publication bias; moreover, sensitivity analysis was used to see how each study impacts the overall estimate of effect.
The bibliographic search was performed up to February 2021 and identified a total of 382 records. A total of 38 duplicate papers and 306 studies were excluded according to the title and inclusion criteria. Subsequently, 14 articles were excluded based on the abstract. A total of 11 papers were excluded from the full text analysis of the 24 remaining articles because they used criteria different to the Nugent and PCR criteria; three were excluded because they analyzed endometrial and not vaginal discharge samples, two because they did not report at least one of the outcome measures and one because it evaluated male infertility exclusively. We obtained a total of seven studies19,33,35,43–46 which were considered suitable, according to the eligibility, inclusion and exclusion criteria (Tables 1–3). Two studies came from the United Kingdom.35,45 two from Denmark19,43 and one each from the United States,46 Egypt44 and France.33 The studies reported patients recruited in IVF centres, regardless of the cause of female infertility.
Prevalence of bacterial vaginosis
Most studies used the Nugent criteria,12 except for Mangot-Bertrand et al.,33 that exclusively used qPCR,13 and two studies by Haahr et al.,19,43 where both methods were used in the analysis. The results of the two studies by this latter author, were subdivided to perform a different analysis according to the diagnostic method used. A total cohort of 1482 patients was obtained where a normal vaginal flora and BV was detected in 1179 and 303 women, respectively. The overall average estimated prevalence of BV in patients with infertility and IVF was 20.4% (Appendix 2). The lowest and highest prevalence was reported by Mangot-Bertrand et al.,33 (9.4%) and by Selim et al.,44 (36.6%), respectively.
|
Author |
Year |
BV analysis method |
n |
Bacterial vaginosis |
% prevalence |
|
Liversedge et al.48 |
1999 |
Nugent |
301 |
77 |
25,6 |
|
Gaudoin et al.56 |
1999 |
Nugent |
212 |
40 |
18,9 |
|
Eckert et al.57 |
2003 |
Nugent |
91 |
10 |
11 |
|
Selim et al.55 |
2010 |
Nugent |
71 |
26 |
36,6 |
|
Mangot-Bertrand et al.46 |
2012 |
PCR |
307 |
29 |
9,4 |
|
Haahr y Jensen et al.32 |
2016 |
PCR |
130 |
36 |
27,7 |
|
Haahr y Jensen et al.32 |
2016 |
Nugent |
130 |
27 |
20,8 |
|
Haahr y Humaidan et al.54 |
2018 |
PCR |
120 |
32 |
26,6 |
|
Haahr y Humaidan et al.54 |
2018 |
Nugent |
120 |
26 |
21,7 |
|
Total |
|
|
1881 |
303 |
20,4 |
Appendix 2 Prevalence of bacterial vaginosis in infertile population
Of the studies that used two diagnostic methods, Haahr et al.,19 detected a prevalence of BV evaluated by the Nugent score and qPCR of 21% and 28%, respectively. The same author in 201943 detected a prevalence of 21.7% with the Nugent criteria. However, this was 26.6% using qPCR. Mangot-Bertrand et al.,33 compared the prevalence of BV with Nugent and qPCR, although the analysis was ultimately performed with qPCR. The latter authors found that the 69 patients classified as intermediate flora by the Nugent criteria were reclassified using qPCR (11 turned out to have BV and 58 were normal). From these latter three studies it appears that the predictive capacity of BV is probably higher with the qPCR analysis than with the Nugent criteria,12 whereby the following meta-analyses were performed overall and according to each diagnostic method used.
Biochemical pregnancy
Five of the seven papers included in the meta-analysis analyzed this variable.19,33,44–46 The average prevalence of biochemical pregnancy in the infertile population was 37.1% (Appendix 3). None of the studies in their individual analysis, detected a significant difference on the rates of biochemical pregnancy with BV, in comparison to those with normal microflora. When performing the overall meta-analysis, according to the analysis of heterogeneity provided by DerSimonian and Laird,42 the interpretation of variance and other heterogeneity indices did not detect apparent evidence of between-study heterogeneity (P=0.78; H2=1, I2=0% and tau2=0). The forest plot revealed that the rate of overall biochemical pregnancy was significantly lower for patients with BV in comparison to those that had normal vaginal flora (OR 0.55; 95% CI: 0.36 to 0.85; P=0.004; Figure 2). However, these results must be interpreted with caution given that significant publication bias was observed in the funnel plot. Moreover, when estimating the impact of each study by means of the sensitivity analysis, a significant variation in terms of the direction and scale of the result (Appendix 4 and Appendix 5) was observed.
|
Author |
Year |
BV analysis method |
n |
Biochemical pregnancy |
% prevalence |
|
Gaudoin et al.48 |
1999 |
Nugent |
212 |
66 |
31,1 |
|
Eckert et al.57 |
2003 |
Nugent |
91 |
41 |
45 |
|
Selim et al.55 |
2010 |
Nugent |
71 |
30 |
42,2 |
|
Mangot-Bertrand et al.46 |
2012 |
PCR |
307 |
109 |
35,5 |
|
Haahr y Jensen et al.32 |
2016 |
PCR/NUGENT |
84 |
38 |
45,2 |
|
Total |
|
|
765 |
284 |
37,1 |
Appendix 3 Prevalence of biochemical gestation in infertile population with bacterial vaginosis

Figure 2 Forest plot of biochemical pregnancy rates in BV patients compared to patients with normal vaginal microbiota. The figure represents the individual ORs with 95% confidence intervals and the combined OR of the fixed effects model and the random effect model. The size of the squares for the individual studies was proportional to the weight of the study.
Given that the use of antibiotics in BV patients could have had an impact on the meta-analysis results, a second analysis was performed excluding the study by Selim et al.,44 A similar result with statistical significance (OR 0.54; 95% CI: 0.34 to 0.85; P=0.007) and without apparent between-study heterogeneity (P=0.65; H2=1; I2=0%; tau2=0; Figure 3), was obtained. Finally, we subdivided the analysis according to type of diagnostic test used to define BV. The forest plot, considering only the studies that used the Nugent criteria,19,44–46 again reflects that there is a significant negative association between BV and rate of biochemical pregnancy (OR 0.55; 95% CI: 0.32 to 0.94; P=0.0028; Figure 4), apparently without between-study heterogeneity, (P=0.58; H2=1; I2=0%; tau2=0). However, the forest plot for studies with qPCR19,33 did not lead to statistical significance for the association of BV and biochemical pregnancy (OR 0.54; 95% CI: 0.28 to 1,06; P=0.07; Figure 5). While there does not appear to be any heterogeneity (P=0.46; H2=1; I2=0%; tau2=0), these results must be interpreted with caution given that the analysis was only performed with two studies, which might not be representative. In both sub analysis the funnel plot and sensitivity analysis revealed significant publication bias and detected significant associations and variations between the different studies (Appendices 6–9), respectively. The quality of evidence was very low for the rate of biochemical pregnancy according to GRADE (Appendix 1).
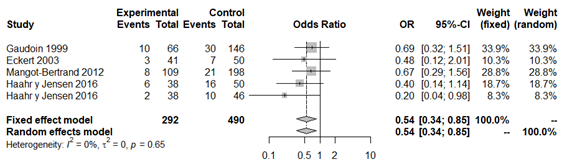
Figure 3 Forest plot of biochemical pregnancy rates in BV patients compared to patients with normal vaginal microbiota, excluding Selim et al.42

Figure 4 Forest plot of biochemical pregnancy rates in BV patients compared to patients with normal vaginal microbiota, according to Nugent criteria.

Figure 5 Forest plot of biochemical pregnancy rates in BV patients compared to patients with normal vaginal microbiota, according to qPCR classification.

Appendix 6 Funnel plot of global biochemical gestation rate without Selim et al.42

Appendix 7 Sensitivity analysis of global biochemical gestation rate without Selim et al.42
Clinical pregnancy
There were five authors that provided information on this variable.19,33,35,43,45 The overall average prevalence of clinical pregnancy in the infertile population was 29.7% (Appendix 10). In most studies, with the exception of two,19,43 no statistically significant difference was detected in the rates of clinical pregnancy of women with BV. Haahr et al.,19 did not find a statistical difference with the Nugent criteria. However, when using classification by qPCR the difference was statistically significant. However, the same work team in 201943 found a statistically significant difference by both analysis methods (Nugent and qPCR), between the groups in the rates of clinical pregnancy. This was less common in cases of BV.
|
Author |
Year |
BV analysis method |
n |
Biochemical pregnancy |
% prevalence |
|
Gaudoin et al.48 |
1999 |
Nugent |
212 |
66 |
31,1 |
|
Eckert et al.57 |
2003 |
Nugent |
91 |
41 |
45 |
|
Selim et al.55 |
2010 |
Nugent |
71 |
30 |
42,2 |
|
Mangot-Bertrand et al.46 |
2012 |
PCR |
307 |
109 |
35,5 |
|
Haahr y Jensen et al.32 |
2016 |
PCR/NUGENT |
84 |
38 |
45,2 |
|
Total |
|
|
765 |
284 |
37,1 |
Appendix 10 Prevalence of clinical pregnancy in infertile population with bacterial vaginosis
The forest plot revealed a statistically significant lower rate of clinical pregnancy in patients with BV (OR 0.43; 95% CI: 0.22 to 0.87; P=0.018; Figure 6). According to the heterogeneity analysis with a confidence level of 95%, at least moderate evidence of heterogeneity was detected (P=0.02; H2=1.59; I2=60%, tau2=0.46), whereby we used the random effects model to interpret results. The funnel plot revealed significant publication bias. Sensitivity analysis detected significant associations and variations between the different studies (Appendix 11 and Appendix 12).

Figure 6 Forest plot of clinical pregnancy rates in BV patients compared to patients with normal vaginal microbiota. The figure represents the individual ORs with 95% confidence intervals and the combined OR of the fixed effects model and the random effect model. The size of the squares for the individual studies was proportional to the weight of the study.
When performing a second overall analysis excluding the study by Liversedge et al.,35 given that patients with BV were treated with antibiotics, the statistically significant relationship was maintained (OR 0.33; 95% CI: 0.16 to 0.70; P=0.003; Figure 7). We highlight that in spite of the relationship demonstrated, the between-study publication bias and moderate heterogeneity remains (P=0.11; H2=1.34; I2=44%; tau2=0.35) (Appendix 13 and Appendix 14).

Figure 7 Forest plot of clinical pregnancy rates in patients with BV compared to patients with normal vaginal microbiota, excluding Liversedge et al.35 for using antibiotics in patients with BV.

Appendix 13 Funnel plot of clinical pregnancy rate without Liversedge et al.35

Appendix 14 Sensitivity analysis of clinical pregnancy rate without Liversedge et al.35
However, the analysis of studies that only used the Nugent criteria12,35,43,45 revealed that there is no statistically significant association between variables (OR 0.58; 95% CI: 0.24 to 1.36; P=0.20; Figure 8), with moderate heterogeneity (P=0.09; H2=1.50; I2=54%; tau2=0.37). Finally, the analysis of studies that only used the qPCR classification19,33,43 did not detect a statistically significant association between variables either (OR 0.29; 95% CI: 0.08 to 1.03; P=0.056; Figure 9), apparently without between-study heterogeneity, (P=0.06; H2=1.69; I2=65%; tau2=0.81). We again have to consider that this latter analysis only included three studies, whereby the result must be taken with caution. In both sub analysis the funnel plot revealed significant publication bias and the sensitivity analysis detected significant association and variations between the different studies, which makes the results less robust (Appendices 15–18). The quality of the evidence of the rate of clinical pregnancy was very low according to GRADE (Appendix 1).

Figure 8 Forest plot of clinical pregnancy rates in BV patients, solely according to Nugent's criteria, compared to patients with normal vaginal microbiota.

Figure 9 Forest plot of clinical pregnancy rates in BV patients, solely according to qPCR classification, compared to patients with normal vaginal microbiota.
Early spontaneous miscarriage
The risk of early loss of pregnancy after pregnancy established in patients with infertility because of BV, was notified in four of the seven studies included in this review.33,35,44,46
The average prevalence of early spontaneous miscarriage in the infertile population at issue was 21.2% (Appendix 19). None of the studies reported statistically significant differences in the rate of early spontaneous miscarriage. The overall meta-analysis of the association between miscarriage in the first trimester and BV led to an OR of 1.13 (95% CI: 0.46 to 2.82; P=0.78; Figure 10) without a statistically significant association. We highlight that no between-study heterogeneity was detected (P=0.70; H2=1; I2=0%; tau2=0). We excluded Liversedge et al.,35 and Selim et al.,44 from a second analysis given that both used antibiotics in patients with BV. The new meta-analysis without them led to an OR of 0.61 (95% CI: 0.34 to 3.09; P=0.54). Once again without apparent between-study heterogeneity (P=0.64; H2=1; I2=0%; tau2=0; Figure 11). Finally, when only analysing studies with Nugent criteria,35,44,46 that is, excluding Mangot-Bertrand et al.,33 similar results were obtained (OR 1,40; 95% CI: 0.34 to 3.84; P=0.51) without probable between-study heterogeneity (P=0.78; H2=1; I2=0%; tau2=0; Figure 12). A meta-analysis with qPCR could not be obtained because only one study for analysis was obtained33. Significant publication bias was again revealed between studies with significant variations among the different studies during the sensitivity analysis (Appendices 20–24). The quality of the evidence was very low according to GRADE (Appendix 1).
|
Author |
Year |
BV analysis method |
n |
Early abortion |
% prevalence |
|
Liversedge et al.48 |
1999 |
Nugent |
88 |
13 |
14,8 |
|
Eckert et al.57 |
2003 |
Nugent |
41 |
14 |
34,1 |
|
Selim et al.55 |
2010 |
Nugent |
30 |
4 |
13,3 |
|
Mangot-Bertrand et al.46 |
2012 |
PCR |
109 |
26 |
23,8 |
|
Total |
|
|
268 |
57 |
21,2 |
Appendix 19 Prevalence of early spontaneous abortion in infertile population with bacterial vaginosis
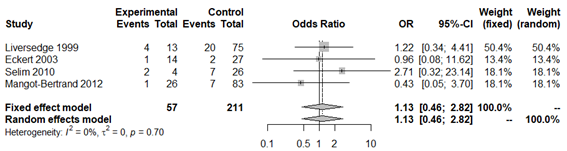
Figure 10 Forest plot of early spontaneous abortion rates in BV patients compared to patients with normal vaginal microbiota. The figure represents the individual ORs with 95% confidence intervals and the combined OR of the fixed effects model and the random effect model. The size of the squares for the individual studies was proportional to the weight of the study.

Figure 12 Forest plot of early miscarriage rates in BV patients, solely according to Nugent's criteria, compared to patients with normal vaginal microbiota.
Live newborns
The final study variable was number of live newborns. Only three of the studies reported this result.33,35,46 The prevalence of live newborns in the infertile population with pregnancy achieved by IVF, was 71.8% (Appendix 25). No statistically significant association was obtained with this variable in the overall analysis (OR 1.63; 95% CI: 0.34 to 4.32; P=0.33; Figure 13) and without apparent between-study heterogeneity (P=0.71; H2=1; I2=0%; tau2=0). However, publication bias was obtained (Appendix 26). In a second analysis without Liversedge et al.,35 virtually the same result was obtained (OR 2.11; 95% CI 0.42 to 10.69; P=0.36; Figure 14). No heterogeneity was revealed (P=0.34; H2=1; I2=0%; tau2=0). The same occurred in the analysis only according to Nugent criteria35,46 (OR 1.32; 95% CI 0.44 to 3.97; Figure 15). Once again no evidence of heterogeneity was obtained (P=0.83; H2=1; I2=0%; tau2=0). For all analyses the funnel plot revealed significant publication bias. Sensitivity analysis revealed statistically significant differences when excluding different studies, which affects the direction and scale of the final result (Appendix 27–29). The quality of evidence of the rate of clinical pregnancy was very low according to GRADE (Appendix 1).
|
Author |
Year |
BV analysis method |
n |
Live births |
% prevalence |
|
Liversedge et al.48 |
1999 |
Nugent |
88 |
70 |
79,5 |
|
Eckert et al.57 |
2003 |
Nugent |
41 |
27 |
65,8 |
|
Mangot-Bertrand et al.46 |
2012 |
PCR |
109 |
74 |
67,8 |
|
Total |
|
|
376 |
284 |
71,8 |
Appendix 25 Prevalence of live births in pregnant women with IVF and bacterial vaginosis

Figure 13 Forest plot of live newborn rates in BV patients compared to patients with normal vaginal microbiota. The figure represents the individual ORs with 95% confidence intervals and the combined OR of the fixed effects model and the random effect model. The size of the squares for the individual studies was proportional to the weight of the study.

Figure 14 Forest plot of live newborn rates in BV patients compared to patients with normal vaginal microbiota, excluding Liversedge et al.,35 since they used antibiotics in BV patients.

Figure 15 Forest plot of live newborn rates in BV patients, according to Nugent criteria only, compared to patients with normal vaginal microbiota.

Appendix 26 Funnel plot of the global live birth rate of pregnant women with IVF and bacterial vaginosis.

Appendix 27 Sensitivity analysis of the global live birth rate of pregnant women with IVF and bacterial vaginosis.

Appendix 28 Funnel plot of the global live birth rate of pregnant women with IVF and bacterial vaginosis, without Liversedge et al.35
For many years it was thought that bacterial vaginosis may have a negative impact on reproductive outcomes of IVF in terms of implantation, gestation and early spontaneous miscarriage.19,33,35,43–46 We performed a meta-analysis with the most recent evidence to evaluate the impact of bacterial vaginosis on these outcomes.
A significant negative association has been revealed globally between bacterial vaginosis and the rate of biochemical and clinical pregnancy. However, this was not observed for the variables early spontaneous miscarriage and live newborns. However, curiously the significant relationship with biochemical gestation was maintained only with the Nugent criteria and not qPCR analysis. In the same way, when subdividing the analysis of clinical pregnancy, no statistically significant association was found with either of the two BV diagnostic methods.
It is known that numerous micro-organisms may not be identified by the Nugent criteria.12 This is because these criteria do not report an exact specimen, but rather classify bacterial communities in accordance with their gram stain morphology.13 This leads to a significant error in the identification of certain pathogenic organisms.45,46 Haahr et al.,19 found that some vaginal communities, such as Lactobacillus iners, are difficult to differentiate from Gardenella vaginalis using the Gram stain. Moreover, there is major variability in interpreting laboratory techniques used by the Nugent score,12 mainly for specimens dominated by L. iners.13,19 According to this author, given the low specificity of the results, the predictive capacity of BV with the qPCR criteria13 is probably greater than that performed with Nugent12 (28% vs. 21%, respectively). For this reason, we also analyzed each variable, according to the type of diagnostic method used for BV.
The standard procedure of many fertility centres includes antibiotic prophylaxis during ovarian puncture. Therefore, we must consider that it is possible that antibiotic prescription has not been explicitly reported in all studies, which could have some impact on their results. Therefore, to avoid any possible bias from antibiotic administration for BV and associated reproductive outcomes, a second analysis excluded Liversedge et al.,35 and Selim et al.,44 The work performed by Eckert et al.,46 was not excluded from the second analysis because the tetracyclins used are not considered as a BV treatment. By excluding these studies there was no variation in the overall result for any variable. We detected important publication bias on all funnel plots. There was in the main moderate heterogeneity among studies and analysis of sensitivity revealed a significant difference in results, in terms of their scale and direction. The reason for the major variability among studies and difficult comparison between these may be due to multiple factors, many of them inherent to each study.
First, as highlighted by other authors previously,17,20,49 the methodology of studies is probably the most important reason. Despite us using studies that only included BV analysis by Nugent or qPCR, there was no consistency to these. Haahr et al.19,43 in their two publications in this regard reported that samples with Gram stain used for Nugent criteria, were reviewed by two different laboratory technicians. When there was a discrepancy between them, they were reviewed by a microbiologist. Liversedge et al.,35 also reported that samples were reviewed by two different technicians. Nonetheless, in the remaining studies the method to analyse and review these samples was not specified. Against the same backdrop, in the qPCR analysis, the thresholds to consider diagnosis of BV were not the same. In their two studies Haahr et al.,19,43 used a level of DNA from Atopobium vaginae >5.7x106 copies/mL and a level of DNA from Gardnerella Vaginalis >5.7x107copies/mL to diagnose BV. However, Mangot Bertrand et al.,33 used a level of DNA from Atopobium vaginae >108copies/mL and a level of DNA from Gardnerella Vaginalis >109copies/mL.
However, all studies included had imprecise definitions that were different for each variable. Biochemical pregnancy was defined by Gaudoin et al.,45 as the existence of a positive test, although without specifying the day. Eckert et al.,44 defined this as a positive test on day 5 of transfer. Selim et al.,44 and Mangot-Bertrand et al.,33 defined it as a positive test on day 15 and finally Haahr et al.,19 as a positive test on day 14. Clinical pregnancy was defined by Liversedge et al.,35 as the existence of a gestational sac at four weeks. The remaining authors defined this as the existence of a foetal pulse although at different weeks of gestation.19,33,43,45
Early miscarriage was defined by Liversedge et al.,35 as the loss of pregnancy during the first trimester. Eckert et al.,46 defined this as a positive titre of human chorionic gonadotrophin (HCG) on day 5, but without a foetal pulse on ultrasound five weeks after transfer. Mangot-Bertrand et al.33 defined this as loss of gestation before week 12. Finally, Selim et al.,44 did not clarify an exact definition. The major variation in definitions means that the authors’ own results are not reproducible and it is not possible to extrapolate their results. In addition, most of the articles did not take into account other factors that could influence the frequency of abortions in patients with infertility and that, therefore, could be part of the bias in their results. For example, Bu et al.,47 found that the age of the woman, the number of previous spontaneous abortions, the thickness of the endometrium on the day of embryo transfer, a history of polycystic ovary syndrome, uterine malformations, and conjugated embryo transfer are independent risk factors for the development of spontaneous abortions. On the other hand, Lambrinoudaki et al.48 found that Glycoprotein IIIa leu33pro polymorphism is associated with early, spontaneous miscarriage, although more studies are needed to validate their results.
However, there was a significant difference at the time of collecting the vaginal discharge sample. In two studies19,43 vaginal samples were collected before IVF stimulation and in five of them during stimulation for one cycle (two during the embryo transfer stage44,46 and three during ovocyte recovery33,35,45). Given that increased oestrogen levels can have an impact on the composition of the vaginal flora,49,50 taking a sample during follicular puncture may have a clear impact on results. The sample should be taken when hormone levels are low, that is, between two and four weeks before starting IVF.51,52
It should also be borne in mind that the studies included come from completely different countries where the prevalence of BV could vary substantially according to some authors.7,53
Finally, another reason for the heterogeneity between studies is that they included patients with different reasons for infertility. The prevalence of BV between them was not differentiated. Two meta-analyses17,20 revealed that the risk of BV is significantly higher in patients with tubal infertility in comparison to patients with non-tubal infertility. It is suggested that bacteria associated with BV probably ascend up the cervix towards the upper genital tract causing inflammation, infection and subsequent tubal damage.
The bibliography includes three meta-analyses in this regard.17,20,54 These highlight once again the main limitation of the major heterogeneity in the methodologies of the studies. In the first meta-analysis performed by van Oostrum et al.,17 an association between the rate of conception and BV could not be confirmed. While it was detected that BV is significantly associated with preclinical loss of pregnancy, an association with early spontaneous miscarriage was not observed. Haahr et al.,20 did not detect a significant association with biochemical or clinical pregnancy either. However, an association with early loss of pregnancy was observed. The explanation suggested for this latter relationship is backed by the fact that during an ascending bacterial infection, associated with BV, implantation could be interrupted.55,56
In the most recent review and meta-analysis by Singer et al.,54 abnormal vaginal flora was significantly associated with lower rates of early development of pregnancy. One of the limitations that has probably had an impact on this outcome is that clinical pregnancy was defined as the existence of a foetal heartbeat and/or a positive human chorionic gonadotrophin result before week 10 of gestation. Both biochemical and clinical gestation are included in the same definition. Perhaps the most important limitation of these meta-analyses is that they included studies with multiple criteria for defining BV. Their results, just as ours, must be interpreted with caution, given that the quality of evidence extracted was classified as very low using the GRADE tool.
Unlike the above studies, our work has the strength that differentiates biochemical from clinical gestation, tries to standardize analysis according to just two of the diagnostic criteria for BV. We performed a second analysis without the studies that used antibiotics in patients with BV and finally, broadly criticized the difficulty to extrapolate results.
It would be interesting to evaluate in the future whether probiotic supplementation significantly improves composition of the vaginal flora and, therefore, the reproductive outcomes of infertile women with IVF. To date there is only one randomized clinical trial57 that reveals that supplementation with L. rhamnosus significantly increases the existence of lactobacilli. Finally, it is still necessary to investigate other risk factors present in this group of women that could be affecting reproductive results, such as the case of Chakra’s energy deficiency. Authors such Huang, suggest that this could be the main cause of decreased fertility and perinatal outcomes in this group of patients.58 In both cases, Further studies are required to confirm or refute this relationship.
Limitations
We recognize that given the sparse bibliography we had limited selection criteria. Having included few studies makes it difficult to extrapolate data. All studies included in this work were observational with relatively small sample sizes and poor quality in general.
There was a broad date range for studies included with a difference of 21 years between the oldest and most recent. The oldest studies may not be as accurate as the most recent studies. Moreover, given that the studies included patients who attended infertility clinics, without defining their characteristics, this population may not represent the infertile population as such and merely represent a subset of infertile women. Finally, we cannot rule out the impact of inherent publication bias.
We demonstrate a negative impact of BV on the reproductive outcomes in IVF, for rates of biochemical and clinical pregnancy. The major between study heterogeneity means further research is necessary. Ideally this would be randomized controlled trials to draw more robust and precise conclusions. We need to standardize methodology by setting out clear definitions for each variable, with a single diagnostic method, by optimizing the time and method of collection of the discharge sample and avoiding the impact of antibiotics or antifungals that can alter the vaginal flora. Finally, it would be interesting in the future to evaluate the preventive effect of probiotic supplements. This is for the purpose of improving the vaginal flora of infertile patients and, therefore, reproductive outcomes.
None.
Author contribution statement: All authors made equal contributions during all phases of drawing up this meta-analysis.
None.
The authors declare no conflicts of interest. This study was not awarded any specific subsidy from any financing agency in the public, commercial or non-profit sector.

©2022 , et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.