eISSN: 2377-4304


Case Report Volume 13 Issue 5
1Hospital Bité Médica, México
2Clínica Helix, ciudad de México, México
3Hospital MAC Periférico Sur, México
4Instituto de Terapia Celular (ITC), Guadalajara, Jalisco, México
5National Academy of Medicine México, México
6Mexican Academy of Surgery, México
7American College of Surgeons, International Fellow, USA
8Banco de Cordón Umbilical (BCU), México
Correspondence: Luján Irastorza Jesús Estuardo, Managing Director, Hospital Bité Médica, Clínica Helix, ciudad de México, México, Tel 5521292609
Received: September 11, 2022 | Published: October 03, 2022
Citation: DOI: 10.15406/ogij.2022.13.00668
In Mexico, 17% of women of childbearing age have infertility problems, this alteration may be associated to Premature Ovarian Insufficiency (POI). On the other hand, Premature Rupture of Membranes (PROM) is defined as the rupture of ovular membranes before labor begins.
The application of Mesenchymal Stem Cells (MSCs) has been proposed for the treatment of POI, placental abruption and PROM.
Objective: Describe a case report of a patient that received MSCs by intravenous injection as an adjuvant for the treatment of POI, and as an aid to reduce placental hematomas that appeared during pregnancy, which resulted in PROM (preterm, and previable), and a preterm baby delivery (alive).
Clinical case presentation: A 30-year-old woman with history of primary infertility of 2 years; Anti-Müllerian Hormone (AMH) of 0.2 ng/mL; laparoscopic Bilateral Tubal Obstruction (BTO), endometriosis, diagnostic and surgical laparoscopy for myomatosis, and Factor VII deficiency; as well as 3 IVF, 4 embryo transfers, and 1 IUI unsuccessful. With confirmed POI diagnosis, the application of several doses at different times of MSCS is decided, resulting in pregnancy. Sometime later during pregnancy, placental hematomas and PROM are observed and decided to apply MSCs at different stages of pregnancy, resulting in the live birth of a baby (29.3 Weeks of Gestation)
Conclusion: The application of multiple doses of MSCs turns more efficient the placental tissue restoration, allowing hematomas to disappear, and delaying a possible PROM.
Keywords: mesenchymal stem cells, anti-Müllerian hormone, premature rupture, placental hematomas
POI, premature ovarian insufficiency; PROM, premature rupture of membranes; MSCs, mesenchymal stem cells; AMH, anti-Müllerian hormone; BTO, bilateral tubal obstruction; FSH, follicle stimulating hormone; LH, luteinizing hormone; WG, weeks of gestation; COS, controlled ovarian stimulation; RDE, retained dead egg; NK, natural killers; TNF-α, tumor necrosis factor alpha; lymphotoxin-alpha; AD-MSCs, adipose tissue derived mesenchymal stem cells; UC-MSCs, umbilical cord-mesenchymal stem cells; BCU, bank of umbilical cord of México
In Mexico, 17% of women of childbearing age have infertility problems, which is equivalent to 1.4 million of couples requiring assisted reproduction techniques,1,2 from which, 9 to 25% of the patients may show a low ovarian response defined as a poor obtention of oocytes after an ovarian stimulation.3,4 This alteration may be associated to Premature Ovarian Insufficiency (POI) characterized by an increase of the Follicle Stimulating Hormone (FSH), and Luteinizing Hormone (LH), with diminution of Anti-Müllerian Hormone (AMH), and estrogens, including menstrual modifications before 40 years old (oligomenorrhea or amenorrhea),5–7 with a prevalence of 1 out of 100 women before 40, and 1 out of 1000 women before 30. The risk varies depending on the race, from 0.1% in Japanese to 1% in Caucasians, and 1.4% in Africans and Hispanics.7–9 Diverse POI etiopathogeneses have been described; i.e. autoimmune diseases,10 oxidative stress,11 genetic predisposition,12 radiotherapy, and chemotherapy in the treatment of cancer.13–15 This can lead to problems of collection and maintenance of primordial follicles, in addition to the induction of follicular atresia, and apoptosis of granulosa cells.16–19 Only 5 to 15% of these patients are able to have a spontaneous pregnancy.20–22
On the other hand, Premature Rupture of Membranes (PROM) is defined as the rupture of ovular membranes before labor begins. They are classified as
The pathogenesis of PROM is uncertain, but it is thought that is caused by a physiological weakening of membranes due to a decrease in the resistance. It is a complication in 3% of pregnancies and causes a 25-30% of preterm deliveries; therefore, it is considered as the main cause of prematurity and maternal mortality. Among the risk factors are PROM in prior pregnancies, genital/intrauterine tract infections, hemorrhages, hematomas, cervical anomalies, invasive procedures, smoking, etc. The management of these patients depends on the fetal maturity. There are 2 types of management:
In the case of POI, there are many situations where the only option for the patient to get pregnant is to accept egg donation,24,25 situation that is not well received by some couples, and as there are no treatment that restores ovarian function, some medical specialists have been prompted to investigate new treatment alternatives. En example of this is the direct application of mesenchymal stem cells (MSCs) via intravenous injection or directly into ovaries. Similarly, intravenous application of MSCs has been proposed for the treatment of placental abruption and PROM when they are caused by the presence of hematomas.26
Because of their high proliferation rate, regeneration, and high degree of differentiation into different cell types through asymmetric divisions,27,28 MSCs in recent years have been considered as a new option to be used in regenerative medicine. MSCs may be obtained from embryonic and extraembryonic tissue, as well as from adult organs, some of which are bone marrow, peripheral blood, umbilical cord, adipose tissue, etc.; in addition, they may be autologous or from donor (allogenic). Their application in clinical research has been as a cellular therapy for conditions such as Alzheimer, lateral amyotrophic sclerosis, Huntington disease, Parkinson disease, cerebral and myocardial infarctions, medullar damage, immune disturbances, arthrosis, restoration of ovarian function, reduction of cerebral hematomas, etc. They release a wide selection of cytokines, chemokines, and growth factors, with anti-apoptotic, anti-inflammatory, proangiogenic, and immunomodulating characteristics; additionally, they are an aid in tissular restoration and replacement of damaged cells, which makes them highly attractive for clinical application.29–32
The following is a case report of a patient that received MSCs by intravenous injection as an adjuvant for the treatment of POI, and as an aid to reduce placental hematomas that appeared during pregnancy, which resulted in PROM (preterm, and previable), and a preterm baby delivery (alive).
A 30-year-old woman with history of primary infertility of 2 years; AMH, 1,9; laparoscopic Bilateral Tubal Obstruction (BTO), endometriosis, diagnostic and surgical laparoscopy for myomatosis, and Factor VII deficiency; as well as 3 IVF, 4 embryo transfers, and 1 IUI unsuccessful, attended the PRONATAL Clinic because she wanted to get pregnant; where, after a background check, a Controlled Ovarian Stimulation (COS) was started with long protocol. It began the 21st day of the previous cycle with Lucrin, 20 IU/24h for 3 days; then, 10 IU/24h, and stopping on the first day of menstruation. That day the application of Merapur, 300IU was started from Day 1 to Day 11, and Choragon, 10000IU on Day 12. Eight MII oocytes were obtained by follicular aspiration, from which 1 developed into blastocyst (BH/BB, euploid), and 7 degenerated.
Therefore, the euploid blastocyst was vitrified and it was decided to accumulate oocytes, which was not possible because the patient presented a spontaneous pregnancy [Retained Dead Egg (RDE) of 10.3WG], where the loss was attributed to inflammation by increased immunity as the patient showed an increment of Natural Killers (NK) cells (12%) in peripheral blood, and Tumour Necrosis Factor alpha (TNF-α) G308A (heterozygote), TNF-α G238A (homozygote) and Lymphotoxin-alpha (LT-α) A252G (heterozygote) polymorphism. So, a treatment was established with Lite vaccination (immunotherapy with paternal lymphocytes), and Enbrel (treatment for autoimmune diseases).
Before starting another COS, it was decided to evaluate AMH again, showing a decrease from 1.9 to 0.2ng/mL, which confirmed POI diagnosis. Then, it was decided to carry out a COS by DuoStim at low doses of Pergoveris daily, and the application of MSCs directly in ovary, and systemically (intravenous injection by the end of the second stage of DuoStim). The first stage began with Pergoveris daily, 150IU/75IU from Day 3 to Day 9; Cetrotide, 0.25mg/day on Days 8 and 9; and Ovidrel, 250µg on Day 9. Three oocytes (MII) were obtained, which were fertilized with semen of the partner diagnosed with Normozoospermia, from which none developed into blastocyst.
The second stage of DuoStim began with Pergoveris daily, 150IU/75IU from Day 15 to Day 21; Cetrotide, 0.25mg/day from Day 18 to Day 21; and Ovidrel, 250µg on Day 21. The day of follicular aspiration 4 oocytes were obtained, from which 3 were MII. These, when fertilized with semen of the partner diagnosed with normozoospermia, did not developed into viable blastocysts. At the end of the second stage of DuoStim, after obtaining the last oocyte, 15 million of Adipose tissue derived mesenchymal stem cells (AD-MSCs) diluted in 3mL of Hartman’s solution (4mL of total vol.) were applied directly in ovaries as an adjuvant for the treatment of POI. Also, 30 million of AD-MSCs diluted in 8 mL of Hartman’s solution were applied systemically (intravenous injection). A total of 60 million of AD-MSCs were applied, which were kindly provided by the Cellular Therapy Institute (ITC, Guadalajara, Mexico) (Figure 1).
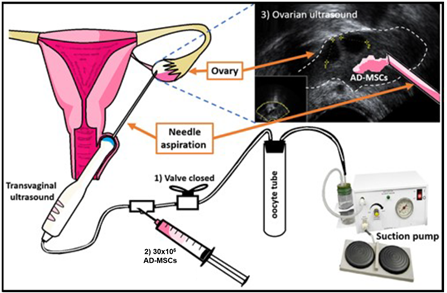
Figure 1 Technique; Application of AD-MSCs in the ovary; 1) The aspiration system is closed to prevent AD-MSCs from going to the oocyte collection tube; 2) AD-MSCs were injected with the flow of the system directed to the ovary, and 3) It is observed whether AD-MSCs are deposited in the ovary.
As the effects of MSCs on ovaries may be observed 2 months after their application;33 within this waiting period, it was decided to transfer the embryo (BH/BB, euploid) obtained in the first cycle carried out in PRONATAL Clinic, which, when devitrified, did not recover well, starting a state of degeneration and cell death resulting in the cancellation of the procedure. This situation disappointed the patients, who decided not to wait for the AD-MSCs to have the expected effect and decided to carry out a cycle with egg donor, where 22 donor oocytes were fertilized with semen of the husband (mild teratozoospermia). Five blastocysts were obtained, from which 3 were vitrified, and 2 were transferred fresh, resulting in a twin pregnancy.
After the patient was able to achieve pregnancy, she had bleeding throughout the first trimester (spotting every other day) since she implanted the embryo.
At 9.3 WG, during obstetrical review an RDE of tween B (Figure 2B), and a 4cm-hematoma in the placenta of embryo A (Figure 2A) were observed; so, it was decided to apply an intravenous injection of 60 million umbilical cord-mesenchymal stem cells (UC-MSCs) [Kindly provided by the Bank of Umbilical Cord of México (BCU)] as an adjuvant to reduce the size of the hematoma.
After application of UC-MSCs, the hematoma was reduced to: 2.41cm (9.5WG), 1.5cm (9.6WG), and 0.8cm (10, and 10.1WG). At 10.1 WG, 35 million UC-MSCs [Kindly provided by the BCU] were applied by intravenous injection as a booster, achieving complete disappearance of the hematoma at 11.5WG (Figure 3).
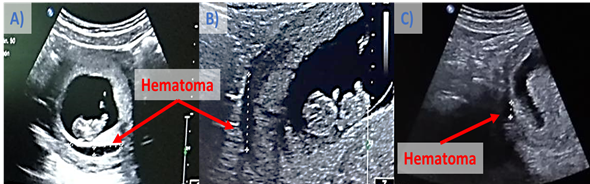
Figure 3 Ultrasound of singleton pregnancy after RDE of Fetus B, in addition to the presence of hematoma that was reduced after application of AD-MSCs. Hematoma size: A) 4 cm (9.3 WG); b) 2.41 cm (9.5 WG), and C) 0.8 cm (10, and 10.1 WG).
At 16.1 WG, the patient arrives to the emergency room, reporting active transvaginal bleeding after sexual intercourse. A suitable implantation was observed by ultrasound; in the upper pole there is an image of hematomas of 1.5 and 1.2cm. In the same way as in week 9.3, the application of UC-MSCs [Kindly provided by the BCU] was made by intravenous injection, as an alternative to reduce hematomas. At 16.3 weeks of gestation, after application of AD-MSCs, hematomas were reduced to 0.4 cm, and 0.3 cm; at 17.6WG no hematomas are observed (Figure 4).
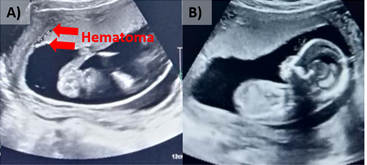
Figure 4 Ultrasound of pregnancy; A) 16.1 WG, 2 small hematomas are observed (1.5 and 1.2 cm), and B) 17.6 WG, with no presence of hematomas.
At 23.2WG, the patient returns to PRONATAL Clinic because a day before she presented red spotting after sexual intercourse (no hematomas are observed). At 28.3WG, the patient reports fluid leakage at night and together with medical history, nitrazine and crystallography test, confirming PROM, therefore, conservative management (expectant) is followed, and 60 million UC-MSCs [Kindly provided by the Bank of Umbilical Cord of México (BCU)] are applied at 29WG.
After application of UC-MSCs at 29.2WG, the lung maturation is completed. Patient's vital signs are stable, amniotic fluid remained stable, hemodynamic parameters normal, and with no data of maternal or fetal inflammatory response. For that moment no maternal or fetal emergency indicated immediate termination of pregnancy; therefore, it was suggested to continue with the conservative management of PROM. Finally, at 29.3WG, cesarean section was performed with the finding of a male birth with 1340g, 41cm, and Apgar 8/9. The child is currently in the first year of life.
Currently, a large number of studies have shown how MSCs help to reduce the symptoms and alterations observed in different pathologies through animal models and clinical trials. This, in turn, has allowed the development of increasingly refined MSCs procurement techniques with ability to isolate such cells from different tissues. In the case of POI, studies of MSCs transplantation have demonstrated their therapeutic potential by restoring ovarian structure and function.34 An example of this is reported by Luján J. et al.,33 who, in a retrospective study that included 8 patients with POI, observed that the application of AD-MSCs increased mean values when the endometrial thickness (8.6 to 9.4mm), the number of oocytes (2 to 9), and their size (13.5 to 15.5mm) were evaluated on Day 11 of menstrual cycle. This allowed to obtain a greater number of MII oocytes (2.6 to 4.2) the day of follicular aspiration, and blastocysts (0 to 3) thereafter.33 Herraiz S. et al.,35 in a group of 17 patients who were poor responders, observed that after application of 50 x 106 BM-MSCs in the ovary by catheterization from Day 2 to Day 43, the number of antral follicles was increased (3 vs 8). Luján J. et al.,2 in a case report of a woman (39 years old) diagnosed with POI, reported that the application of AD-MSCs (intra-ovarian) increased the mean number of MII oocytes obtained (3 to 14); also, Herraiz S. et al.,36 reported an increase of the number of MII obtained (15 vs 30) in immunodeficient mice with ovarian damage induced by chemotherapy, after the application of BM-MSCs; and Li J. et al.,37 in a group of female mice with POI induced by cyclophosphamide, showed an increased number of follicles (11 vs 13) after 6 weeks of application of MSCs from chorionic plate. Unfortunately, in the present work the patients decided not to wait the 2 months indicated in studies before the effects of the application of AD-MSCs can be seen, accepting egg donation.
On the other hand, in this report the patient developed placental hematomas twice after achieving pregnancy by egg donation. The first one at 9.3 weeks of gestation, that was reduced and disappeared approximately at 11.5WG, after application of UC-MSCs. Similarly, at 16.1WG, two hematomas were observed (1.5, and 1.2cm), which also reduced their size and disappeared at 17.6 WG, after application of UC-MSCs. In addition, PROM was present at 28.3WG, and the patient was kept under PROM expectant management. Immediate application of UC-MSCs was carried out, which probably allowed to delay delivery up to the 29.3 WOG, and to complete the lung maturation. Similarly, in a case report by Luján J. et al., 2020, a retro placental hematoma was observed at 25 weeks of gestation (2.8x2.1x0.6cm), which, after 3 applications of AD-MSCs (1st: 25 WOG, 2nd: 26.5 WOG, and 3rd: 27.4WG) was reduced, confirming repair of placental abruption at 28.2 WOG, and resulting in live birth of newborn at 30.1 WOG. This could be due to the fact that the application of MSCs improves the microenvironment surrounding the damaged tissue (hematoma), thus stimulating the generation of new blood vessels, regulating the immune response and preventing cell death by apoptosis, by producing cytokines and growth factors that regulate these processes, in addition to replacing the damaged tissue with new tissue by differentiating.38–41
Finally, there are very few reports of MSCs application in humans, and the mechanisms proposed about how they function have been developed through studies on laboratory animals, which make these procedures an area of opportunity in clinical practice for the study of diverse pathologies such as POI, and PROM, areas in which it is necessary to carry out randomized, controlled trials with large samples.
MSCs transplant studies have demonstrated their therapeutical potential for restoration of ovarian structure and function. Unfortunately, in this work the patients’ decision to accept egg donation after Application of AD-MSCs, prevented us from seeing the potential benefits of AD-MSCs as an adjuvant for the treatment of POI.
The application of multiple doses of UC-MSCs (by intravenous injection) turns more efficient the placental tissue restoration, allowing hematomas to disappear, and delaying a possible PROM. This makes it possible to finalize, as in this case, the lung maturation.
Finally, conservative treatment for placental hematomas and PROM is currently limited, and there is no complementary therapy beyond maternal and fetal surveillance. Therefore, we suggest the development of a protocol for the application of MSCs in PROM, to confirm reproducibility of research findings and to be able to establish this measure as a standard therapeutic option.
None.
None.
Author declares there is no conflict of interest exists.

©2022 , et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.