eISSN: 2377-4304


Case Report Volume 13 Issue 4
1Department of Obstetrics and Gynecology, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain
2Primary Care Interventions to Prevent Maternal and Child Chronic Diseases of Perinatal and Developmental Origin Network (RICORS, RD21/0012/0001), Instituto de Salud Carlos III, Madrid, Spain
Correspondence: Cristina Trilla, Prenatal Diagnosis Unit, Hospital de la Santa Creu I Sant Pau, C/Sant Quintí 89, 08025, Barcelona, Spain, Tel +0034935337041
Received: July 18, 2022 | Published: August 05, 2022
Citation: DOI: 10.15406/ogij.2022.13.00655
Background: Vasa previa consists in the presence of extraplacental fetal vessels overlying the cervix. This condition is commonly associated with fatal outcome if prenatally undiagnosed. Two types of vasa previa have been classically described, associated with well-established risk factors. However, a rare third type of vasa previa has also been reported.
Case: We present a case of type 3 vasa previa diagnosed in the second trimester, with two fetal vessels overlying the cervix. The placenta was unilobed, not previa, and the cord insertion was marginal. Placental examination after delivery confirmed the prenatal findings.
Conclusion: This case suggests that vasa previa can occur in the absence of cord or placental anomalies. A review of vasa previa classification and screening strategies for this condition is needed.
Keywords: vasa previa, prenatal diagnosis, prenatal screening, neonatal outcome
Vasa previa is a rare entity with a fatal outcome if undiagnosed prenatally.1 Classically, two types of vasa previa have been described.2 Type 1 vasa previa is associated with a velamentous cord insertion, where one of the umbilical vessels runs over the cervix to reach the placenta. Type 2 vasa previa is associated with a bilobed or succenturiate lobed placenta, where the vessel connecting the two lobes of the placenta overlies the cervix. Recently, a third type of vasa previa has been reported,3 including cases with a normal cord insertion and a single placental lobe and thus, not fitting the above-mentioned classification.
Undiagnosed vasa previa is commonly associated with fatal fetal outcome. However, prenatal diagnosis of such condition improves fetal outcome in most cases. Several risk factors have been described in association with vasa previa. Targeted screening strategies have thus been suggested to allow prenatal diagnosis in such cases and prevent unfavorable outcomes.4 We present a case of prenatal diagnosis of type 3 vasa previa and discuss potential screening strategies.
Here, we present the case of a nulliparous, 37-year-old patient diagnosed with type 3 vasa previa at 20+2 weeks’ gestation. This was a spontaneous pregnancy in a patient with no medical conditions who presented at the anomaly scan. The fetal anatomy was normal, and the location of the placenta was anterior, not previa nor low-lying. The cord insertion was marginal at the inferior edge of the placenta. A transvaginal ultrasound was performed for the measurement of the cervical length for preterm birth risk assessment and vasa previa screening, according with our institutional protocol. A large vessel crossing over the internal cervical os was identified. The placenta and cord insertion were re-examined, confirming a single placental lobe and a marginal cord insertion. The case was thus oriented as type 3 vasa previa and monthly follow-up ultrasounds were scheduled. Two vessels overlying the cervix were identified at 28 weeks’ gestation (Figure 1). Both vessels emerged from the placenta, close to the cord insertion, and followed a posterior-lateral right direction while crossing over the cervix. The larger vessel showed a venous flow, and the smallest vessel showed an arterial flow (Figure 2) (Figure 3). Further antepartum surveillance included weekly ultrasounds and non-stress tests (NST) from 32 to 36 weeks. The patient was asymptomatic, and the cervical length remained stable between 31and 33mm. Amniotic fluid index and fetal growth were normal, and NST showed a normal fetal cardiac frequency and pattern, as well as absence of uterine contractions.
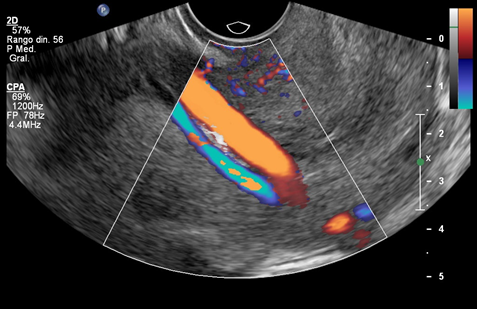
Figure 1 (a) Vasa previa with two fetal vessels overlying the internal cervical os. (b) Power Doppler depicting a marginal cord insertion.

Figure 2 Transvaginal ultrasound at 20 weeks’ gestation in a case of type 3 vasa previa. Color Doppler image showing a large vessel running over the internal cervical os and pulsed wave Doppler evaluation of this vessel, suggesting a venous waveform.

Figure 3 Transvaginal ultrasound in a case of type 3 vasa previa. Color Doppler image showing a smaller vessel running over the internal cervical os and pulsed wave Doppler evaluation of this vessel, suggesting an arterial wave form.
An elective caesarean section was performed at 37+0 weeks. A single course of corticosteroids for fetal lung maturation was administered 4 days before surgery. A girl weighing 2915g was born, with a 5- and 10-minute Apgar score of 10, and umbilical arterial and venous pH of 7.31 and 7.37, respectively. Both maternal and neonatal post-delivery courses were uneventful. The placental examination confirmed the prenatal ultrasound findings. The placenta was unilobed, and the cord insertion was marginal. Two umbilical vessels -one vein (in blue) and one artery- emerged from the lateral side of the cord insertion, following a large extraplacental intramembranous course and merging back into the placenta at the opposite edge (Figure 4). The patient gave her written consent to publish her case, including all accompanying images.
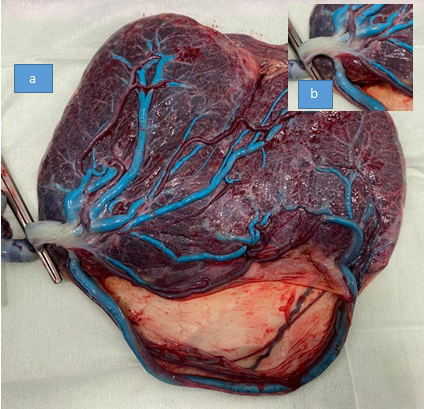
Figure 4 (a) Inspection of the placenta at delivery. An arterial and a venous (in blue) vessel emerge laterally to the cord insertion, follow a large transmembranous course, and merge back into the placenta at the opposite edge, confirming the diagnosis of type 3 vasa previa. (b) Macroscopic confirmation of marginal cord insertion.
The indication for vasa previa screening remains a controversial issue.5,6 Although the prevalence of the condition is low, estimated at 0.60 per 1000 pregnancies,7 its consequences if prenatally undiagnosed can be fatal for the fetus, including a high mortality rate and long-term consequences of acute hypoxia.1,8 Arguments against universal screening include its low incidence and the burden of false-positive diagnosis on maternal and perinatal outcomes.9 However, it has been consistently reported that prenatal diagnosis with color or power Doppler is feasible and accurate9 and has the potential to avoid most neonatal deaths due to vasa previa. Thus, effective targeted screening strategies have been recently proposed based on the recognized risk factors for vasa previa.10
Risk factors for vasa previa include velamentous cord insertion, second-trimester placenta previa or low-lying placenta, conception by in-vitro fertilization, bilobed placenta and umbilical cord insertion in the lower third of the uterus at first trimester ultrasound.7 However, the evaluation of some of these risk factors is not systematically included in prenatal surveys. For instance, many official guidelines do not recommend to routinely assess the placental cord insertion at first or second trimester.11,12
In our case, none of the reported risk factors for vasa previa was identified, although cord insertion was only assessed at the second trimester ultrasound. Hence, we cannot provide information for this factor in first trimester and therefore it is possible that our patient had a low cord insertion at that time. However, type 3 vasa previa, as described by Suekane et al.,3 is not associated with placental or cord anomalies. In the view of the case here described, we believe there is a need for this new category. A marginal cord insertion was noted in our case. Until now, only velamentous cord insertions have been considered as risk factors for vasa previa. Our observations suggest that it is likely that marginal insertions can also constitute a predisposing situation for vasa previa.
In view of the different types of vasa previa and the proposed strategies for screening, several aspects should be considered. First, identifying risk factors for such condition is a valuable clinical approach for screening. However, these factors must be thoroughly and systematically evaluated in first and second trimester ultrasounds in order to guarantee the effectiveness of targeted screening strategies. Second, transvaginal ultrasound for cervical length measurement for preterm birth prevention has become current practice in several clinical settings such as ours.13 We suggest that, if a transvaginal scan is already being performed for this indication, universal vasa previa screening should also be implemented. It is important to note that, in our case, only vaginal examination with color Doppler allowed a rightful diagnosis and an uneventful fetal and maternal outcome.
To conclude, in view of the current knowledge on vasa previa risk factors, the accuracy of prenatal diagnosis and effectiveness of the proposed screening strategies, we believe it is time to consider implementation of vasa previa screening. Whether a targeted strategy based on risk factors or a universal strategy, combined with preterm risk assessment, is preferable in terms of perinatal outcome and cost-effectiveness has yet to be determined. Finally, further research should determine which risk factors are associated with this new classification of vasa previa.
None.
No funding sources were necessary to perform this research.
No potential conflict of interest was reported by the authors.

©2022 , et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.