eISSN: 2377-4304


Research Article Volume 12 Issue 3
1Pronatal Clinic (Bité Médica Hospital), México
2Female Health Clinic, México
Correspondence: Luján Irastorza Jesús Estuardo, Pronatal Clinic (Bité Médica Hospital), México, Tel 5521292609
Received: May 05, 2021 | Published: June 1, 2021
Citation: Estuardo LIJ, Carlos DM, Daniela AR, et al. Prevalence of TNFa (G308A and G238A) and LTa (A252G) polymorphisms in women with pregnancy loss ‒ study carried out in a private clinic of Mexico City. Obstet Gynecol Int J. 2021;12(3):183-188. DOI: 10.15406/ogij.2021.12.00573
Background: Tumor necrosis factor (TNF) is a cytokine that includes different types of molecules that participate in cellular and organic responses, and Single Nucleotide Polymorphisms (SNPs) in TNF are associated to the pathogenesis of chronic inflammatory diseases and local or systemic autoimmune diseases.
Objective: To know the prevalence of TNFα (G238A and G308A) and LTα (A252G) polymorphisms in a population of Mexican women with pregnancy loss.
Materials and methods: This is a retrospective, observational and cross-sectional study of 184 Mexican women, with the aim of evaluating the presence of TNFa (G238A and G308A) and LTa A252G polymorphism; 3 groups were formed: 1) TNFa G238A, 2) TNFa G308A and 3) LTa A252G and each group was separated by homozygous and heterozygous mutation.
Results: It was found an increase in prevalence in TNFa, G238A compared with TNFa G308A and LTa A252G (31.9 vs 25.4 and 26.5%). The heterozygous form was higher in prevalence compared with the homozygous. In 50.3% no mutations of TNFa G238A, TNFa G308A and LTa A252G were found; the number of patients that only presented one polymorphism was 23.2%, with 2 polymorphisms represent 21%, and presented 3 polymorphisms (5.3%).
Conclusion: The prevalence of TNFa G238A, TNFa G308A and LTa A252G polymorphisms in Mexican population could be high. Said polymorphisms are associated to almost 50% of cases of women with pregnancy loss in this study; and patients with more than one polymorphism are susceptible to complications such as pregnancy loss.
Keywords: tumor necrosis factor, pregnancy, cytokine, single nucleotide polymorphisms
TNF, tumor necrosis factor; SNPs, single nucleotide polymorphisms; LTa, lymphotoxin a; PL, pregnancy loss; RT-PCR, reverse transcriptase-polymerase chain reaction; GW, gestational weeks; SD, standard deviation; BMI, body mass index; RPL, recurrent pregnancy loss; IUGR, intrauterine growth restriction
The Tumor Necrosis Factor (TNF) is a cytokine first identified in sera of mice treated with lipopolysaccharides as a mediator of tumor necrosis by inducing hemorrhagic necrosis, and regression of transplanted tumors. It has also been related to the death of tumoral cells in vivo. There are different types of TNF including TNFa and TNFb [Lymphotoxin a (LTa)]. These two cytotoxins are produced mainly by activated macrophages and monocytes, in the case of TNFa, and by lymphoid cells in the case of LTα. Both of the cytokines may communicate in a paracrine, autocrine, or systemic way; so, they may participate in a great variety of cellular and organic responses, including fever, tissue damage, tumor necrosis, anorexia, cell proliferation and differentiation, as well as apoptosis.1–4
Several studies have described the relationship between single nucleotide polymorphisms (SNPs) of TNF and chronic inflammatory diseases, and local or systemic autoimmune diseases such as rheumatoid arthritis, systemic erythematosus lupus, Graves’ disease, diabetes mellitus type I, multiple sclerosis, and pregnancy complications.5,6
In pregnancy, the immune system plays an important roll. The normal development thereof is the result of a balance between the cytokines produced by TH1 immune response (TNFa, TNFb, IFNg, IL2, etc.), and those produced by TH2 immune response (IL4, IL5, IL6, IL 10, IL13, etc.).7–10 A disruption of said balance may cause alterations before and during pregnancy. The Single Nucleotide Polymorphisms of TNFa G238A, and G308A, that modify TNFa and LTα concentrations are an example of this, which, in turn, in many studies have been associated to an increase of pregnancy diabetes, spontaneous abortion, pregnancy loss, recurrent pregnancy loss, preeclampsia, and premature delivery.10,11,12–19 Similarly, an increase in TNFa may induce apoptosis of placental cells, and an inappropriate spiral artery remodeling, in addition to an increase in the risk of placental thrombosis.20–22 It has been documented that cell activation or damages in vascular epithelium cells that stimulate the formation of blood clots, and therefore, thrombi, caused mainly by an increase in TNFa, may be observed in preeclampsia.23,24 In addition to these direct effects, it has been described that TNFa acts indirectly to induce spontaneous abortion through the activation of NK cells and macrophages.25 On the other hand, LTα has a great deal of immunomodulator functions similar to those of the TNFa.26 For this reason, some authors associate the development of pathologies caused by a polymorphism-related increase in TNFa with the increase in LTα caused by the SNP of LTα A252G, as shown by studies that found pregnancy loss related to the expression of the mutations TNFa G238A, and LTα A252G in women.27,28 This relationship might be due to the fact that these two cytokines show similar structures having affinity by the same receptors (TNFR1 and TNFR2).29
Because of the importance of the information below, this study was focused to make known the prevalence of polymorphisms TNFa (G238A and G308A), and LTα (A252G) in women with pregnancy loss attended at PRONATAL clinic in Mexico City.
This is an observational retrospective cross-sectioned study that included 184 women of reproductive age with history if pregnancy loss (PL) attended at the Pronatal clinic between 2015 and 2018, and to whom a study to determine the presence of polymorphisms TNFα (G238A and G308A), and LTα (A252G) was made.
With the data obtained 3 groups were formed:
Additionally, each group was classified by homozygous or heterozygous mutation to carry out an evaluation. Age, weight, and size data were collected in the first visit by the nursing staff.
Mutation analysis was made by taking blood samples that were sent to the Institute of Human Reproduction Science (CDMX, Mexico) where it was determined whether they showed one or more of the SNPs of TNFa (G238A and G308A) and LTα A252G, by means of a Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR).
All patients were informed about the use and management of the data collected (age, weight, size, and results of polymorphisms), which allowed us to include them is this study. Anonymity was kept by not to making reference to the origin of data and making known statistical numerical data only (as the case might be).
Inclusion criteria: Women of reproductive age with complete history (age, weight, size and detection of SNPs of TNFa (G238A and G308A), and LTα (A252G), with or without reproduction issues, and with a background of ≥1 PL before 20 gestational weeks (GW), PL [spontaneous abortion and dead egg retention (DER)], with or without the presence of (hereditary) thrombophilia, study of SNPs of TNFa (G238A and G308A), and LTα (A252G) polymorphisms (negative or positive), and detection of FVL-G1691A, PT-G20210A, MTHFR-C677T and PAI-1 4G/5G (negative or positive).
Exclusion criteria: patients that did not accepted to be included in the study, pregnancy loss associated to infectious, anatomical, immunological, and endocrine factors.
Statistic analysis: Age, weight, and size, are reported as the mean±standard deviation (SD), and the difference of incidence between the different polymorphisms and the homozygote vs heterozygote status was determined by chi-squared tests using the statistics software SPSS ver. 25.
Table 1 shows the data of age, weight, size, and body mass index (BMI) of the 184 women included in the study.
Population characteristics |
|
Age (Years) |
33.6±5.1 |
Weight (Kg) |
60.7±10.2 |
Height (cm) |
167.8±6.3 |
BMI (Kg/m2) |
21.5 |
Table 1 Population age, weight, height, and BMI ( ± DE)
Graph 1 shows that there is no statistically significant difference between the prevalence of TNFa G238A, TNFa G308A, and LTa A252G, but a numerical increase may be observed for TNFa G238A compared with TNFa G308A, and LTa A252G (31.9 vs 25.4 and 26.5%).
When data are analyzed by homozygous vs heterozygous status, numerically, heterozygous status has a higher prevalence compared with homozygotes for TNFa G2238A (14.1 vs 17%), TNFa G308A (10.8 vs 14.6%), and LTa A252G (6.5 vs 20%), from which only LTa A252G polymorphism showed a statistically significant difference (6.5 vs 20%, p<0.05) (Graph 2).
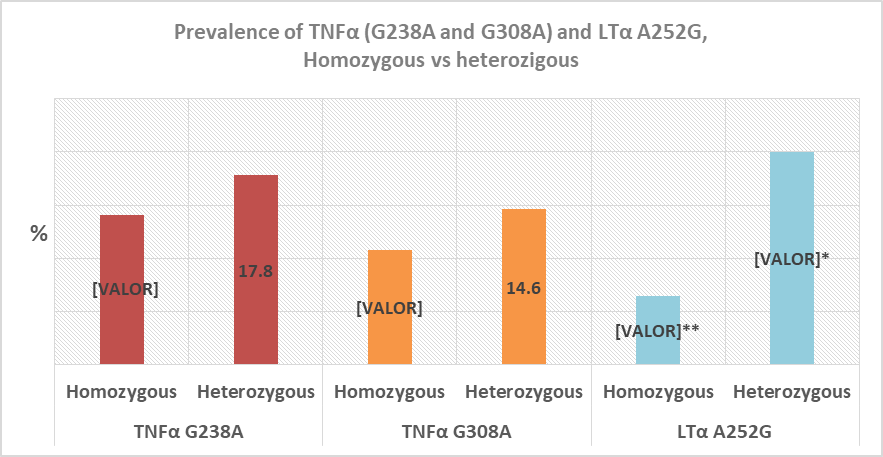
Graph 2 Prevalence of TNFa G238A, TNFa G308A, and LTa A252G polymorphisms in the homozygous and heterozygous state.
*Difference of prevalence between homozygous LTa A252G, and heterozygous LTa A252G
**Difference of prevalence between homozygous LTa A252G vs TNFa (homozygous G238A and G308A). Chi-squared, p<0.05.
When the prevalence of the number of polymorphisms by patient was analyzed, 50.3% did not show any of the TNFa G238A, TNFa G308A, or LTa A252G mutations. As for patients with only one polymorphism, they constituted 23.2% with a prevalence of the several homozygous and heterozygous combinations from 0.5% to 7%. In addition, patients with two polymorphisms constituted 21% with a prevalence of the several homozygous and heterozygous combinations from 0.5 to 3.8%; and finally, 5.3% of the patients showed 3 polymorphisms, with a prevalence of the several homozygous and heterozygous combinations from 0.5 to 2.7% (Graph 3).
There are many studies that report the importance and prevalence of TNFa polymorphisms associated to diverse pathologies at international level. However, in Mexico there is not a representative sample yet that indicates the trend of the TNFa G238A, TNFa G308A, and LTa A252G polymorphisms. This fact remarks the relevance of the present study, which aims to determine the prevalence of these three polymorphisms in patients that attended for obstetric revision and assisted reproductive techniques (ART) to PRONATAL Clinic in Mexico City (CDMX).
A homozygous (14.1%) and heterozygous (17.8%) TNFa G238A polymorphism prevalence was observed in this study, in addition to a homozygous (10.8%) and heterozygous (14.6%) TNFa G308A polymorphism prevalence in women with ≥1 PL. In scientific literature there are studies that have shown prevalences of the homozygous TNFa G238A polymorphism from 0 to 8%, and the heterozygous one from 3 to 31%; and the homozygous TNFa G308A polymorphism from 0 to 12.3%, and the heterozygous one from 6.6 to 48%.11,27,30–45 Many of these studies make reference to the relationship between recurrent pregnancy loss (RPL) and TNFa G238A and TNFa G308A polymorphisms, where Sudhir N,11 in a clinical study of 115 women from 21 to 44 years old, reported a higher rate of TNFa G308A in women with RPL compared with a control group (29.57% vs 16.22%). Jung A,34 in a meta-analysis of 21 studies with 3437 cases and 4016 controls, compared several TNFa polymorphisms with RPL and found an association of TNFa G308A with a higher risk of RPL in the heterozygous form. Similarly, Hui L,46 in a meta-analysis that included 1430 patients with RPL, and 1727 controls, reported that the TNFa G308A polymorphism is correlated to a high risk of RPL in Asian women. As for TNFa G238A polymorphism, Lee B,43 in a study that included 357 Korean women and 236 controls, found that the TNFa G238A polymorphism was associated to an increase in the risk of RPL. Additionally, Zammiti W,28 in a study including 372 women with RPL, found a higher prevalence of TNFa G238A in these patients compared with the control group (23.6 vs 18.9), as well as Finan R., (2010) who observed a higher prevalence of TNFa G238A in 204 women with RPL compared with the control group.
As for the homozygous LTa A252G polymorphism, this study showed a homozygous prevalence of 6.5%, and a heterozygous prevalence of 20%, which is different to the findings of Zammiti W,27 that showed a homozygous LTa A252G prevalence of 21.4%, and a heterozygous prevalence of 4.6% in 350 patients. This increased prevalence was associated to a higher risk of pregnancy loss.27 Additionally, because of their importance during pregnancy, there are studies that have associated the TNFα G308A, TNFα G238A, and LTα A252G polymorphisms to a higher risk of preeclampsia, preterm labor, gestational diabetes, and intrauterine growth restriction (IUGR).47–51
Besides, when the presence of these polymorphisms was evaluated per patient, we observed that 23.2% of them showed one polymorphism; 21%, 2 polymorphisms; and 5.3%, 3 polymorphisms, which constitutes one more reason to justify the study of said polymorphisms’ prevalence, as the presence of more than one polymorphism might increase the possibility to develop the before-mentioned pathologies. Something similar happens in a study carried out in 119 pregnant women with history of venous thrombosis. In that study, the risk of venous thrombosis with the presence of a thrombophilia by G20210A prothrombin or FVL, was compared with the presence of a combination of these two thrombophilias. The highest risk was observed when the patients showed both of the thrombophilias.52 On the other hand, Coulam C,53 observed that 74% of 42 women with history of implantation failure showed more than 3 thrombophilias from the 10 thrombophilias assayed, compared with a control group (20%), and concluded that the association of thrombophilias with implantation failure is manifested by the total number of mutations.
Accordingly, the previous evidence points out to a higher risk of RPL and other conditions that might threaten the life of the mother and the fetus during pregnancy, when said women show TNFa (G238A and G308A), and LTa A252G polymorphisms. This, in addition to the higher rate of RPL (47%) with idiopathic causes,54 gives a high clinical value to the fact to know the prevalence of TNFa (G238A and G308A), and LTa A252G polymorphisms in Mexican population.
Limitations of the study
The number of patients is not representative of Mexican population as these are data collected from just one health center. Even so, we think that the data obtained are of great importance to elucidate the trend in Mexican population.
Fifty percent of Mexican population might show single or combined TNFa G238A, TNFa G308A, and LTa A252G polymorphisms if they show the same pattern that the population that attended to Pronatal Clinic.
With respect to what is described in the literature, 49.7% of the population that attended to Pronatal Clinic might show a higher risk of RPL.
Patients with more than one TNFa polymorphism might be more susceptible to complications that result in pregnancy loss.
It is necessary to carry out a prospective study with a control group that allows evaluating TNFa G238A, TNFa G308A, and LTa A252G polymorphisms in PL and RPL as independent factors in Mexican population.
None.
None.
The author declares that there is no conflict of interest regarding this study.

©2021 Estuardo, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.