eISSN: 2377-4304


Research Article Volume 13 Issue 4
1College of Medicine, King Saud University, Riyadh, 11523, Saudi Arabia
2Anova Fertility and Reproductive Health, Toronto, M2N 6S6, Canada
Correspondence: Murid Javed, Anova Fertility and Reproductive Health, Toronto, M2N 6S6, Canada, Tel +1-416-731-9885
Received: August 07, 2022 | Published: August 29, 2022
Citation: DOI: 10.15406/ogij.2022.13.00660
Embryo implantation is the most important event in the achievement of conception. In the presence of any endometrial disease, this process can be hampered. The endometriosis is linked to causing infertility. It is a chronic uterine disease that is dependent on estrogens and is associated with reduced fecundity. The objective of this study was to investigate the impact of endometriosis on embryo implantation in patients undergoing IVF. This is a case-control study, with case to control ratio of 5:1. The study included 50 patients with endometriosis and 10 patients without endometriosis served as control. The endometriosis was diagnosed by symptoms, pelvic and transvaginal ultrasound examinations. The serum estrogen levels, fertilization rate and implantation rate were determined. Since the presence of a haemorrhagic cyst was suspected at the ultrasonographic finding of masses parallel to the ovaries, measurement of the CA 125 marker was carried out for differential diagnosis. The data were recorded in Excel sheets and analysed using statistical functions of Excel. The significance level was set at 0.05%. Most of the patients in endometriosis group (68%) had elevated CA125 Levels and 56 % had high E 2 level. In the control, only one patient had high E2 level. In the endometriosis group, 31.67% had positive pregnancy test, while 90% patients without endometriosis had positive pregnancy test. These differences were statistically significant. These data reveal that the patients with endometriosis had significantly higher levels of E2 and CA125 marker in blood and had significantly lower implantation rates as compared to those in the control group.
Keywords: Endometriosis, implantation, IVF, pregnancy, pelvic examination, vaginal ultrasound
Endometriosis is a common disease that affects 6 to 10% of woman of childbearing age. It is characterized by growth of endometrial tissue outside the uterine cavity. The deep infiltrating endometriosis is a progressive and hormone-dependent disease with estrogen dependence and progesterone resistance. The main symptoms are dysmenorrhea, chronic pelvic pain, dyspareunia and infertility.1 The endometriosis is subdivided according to the type and location of the lesions into peritoneal endometriosis, deep endometriosis and endometrioma.2
Many factors are believed to cause this condition. The leading theory is retrograde menstruation in which the menstrual blood flows into the abdominal cavity. The viable cells in this flow can implant, cultivate, and penetrate the peritoneal cavity. The retrograde menstruation is a phenomenon that is somewhat normally seen in a large fraction of females of reproductive age.3
The embryo implantation is the most important event in achieving pregnancy. In endometriosis, this process is affected.
This study investigated the impact of endometriosis on embryo implantation in Saudi patients undergoing in vitro fertilization procedures, find ways to improve its diagnosis and to recommend guidelines to reduce its effects on the patients.
The infertility is failure to achieve a clinical pregnancy after 12 months or more of regular unprotected sexual intercourse. Its incidence is increasing worldwide, and endometriosis is one of the common causes of infertility. The incidence of endometriosis in the World is about 8 to 12%.4 Previously published reports found that endometriosis is a cause of infertility.5,6 The endometriosis infertility is due to endocrine abnormalities, fibrosis, adhesions, immune and inflammatory disorders. The females suffering from endometriosis usually have poor growth of ovarian follicles, thus produce low number of oocytes despite high dose of follicle stimulating hormone, and achieve low pregnancy rate.7,8 Many studies have focused on factors affecting the success rate in in-vitro fertilization procedure, however, there is need to explore the success rate after in-vitro fertilization procedure in females suffering from endometriosis, especially in Kingdom of Saudi Arabia.11
Therefore, the aim of this study is to review the current knowledge regarding endometriosis, and efficiency of embryo implantation in IVF procedures. Some studies explored embryo implantation in IVF cycles in endometriosis.12–14 Zhong et. al., studies 330 patients undergoing IVF or ICSI. They concluded that endometriosis causes a harmful impact on the outcomes of IVF and ICSI and the better control and management of endometriosis improved live birth rate.14
Sanchez et. al. reported similar fertilization and quality of embryos but a reduced pregnancy rate in women previously operated for moderate/severe endometriosis as compared with non-affected women.15 In a study to determine the prevalence of endometriosis in women who had gynecologic laparoscopy at a university hospital in Jeddah, Saudi Arabia between January 2008 and December 2013, Rouzi et al. reported 11.1 % incidence of endometriosis.11
The mechanism that leads to failure of reproduction in women suffering from endometriosis is still not clear. One of the options is that the endometrial tissue generates progesterone, oestradiol and cytokines that create inflammatory condition and enhance apoptosis in granulosa cells harming fertility. Additionally, endometriosis causes adhesions in the reproductive system resulting in mechanical interferences for ovulation and fertilization. It has been reported that 30–50% of women diagnosed with endometriosis are infertile.16
Endometriosis and endometrial polyps are also the key contributors to the reduction of female fertility, however, with the introduction of IVF technology, a lot of infertile couples with polyps or endometriosis can conceive and enjoy childbirth. Although, a dubious situation is still hanging whether these assisted reproductive technologies can resolve all infertility-associated concerns of.
Additionally, patients with endometriosis mostly have genetic abnormalities associated with apoptosis, adhesion, wound, and healing reaction.17 It also produces aberrations in the processes where morphological and functional alterations within the endometrium take place to make the decidual lining for the implantation of the blastocyst, a process known as decidualization. All of this largely leads to the failure of the implantation of embryos.18
In fact, the condition of endometriosis reduces a female’s fertility by influencing the quality of egg and embryo. A low rate of fertilization in endometriosis and more atypical eggs (oocytes) in comparison to patients with no endometriosis in the control group. They linked this to the abnormality of steroids in endometriosis patients.19
Estrogen, progesterone, and sex related steroids are largely synthesised in the ovaries and they control the development of endometrial tissue, essentially by activating and preventing proliferation of cell. Estrogen also has vital function in the discharge of gonadotropin and in the formation of follicles.20 There are four different varieties of estrogen, namely estrone (E1), oestradiol (E2), estriol (E3), and estetrol (E4). E2 has a major function in the female reproductive tract.21
The estrogen hormone is responsible for the growth and regulation of the menstrual cycle and female reproductive system. In addition, estrogen plays a vital role in various other biological systems such as immune systems, vascular system, skeletal, and neuroendocrine system. For this reason, estrogen is implicated in problems like infertility.
Estrogen plays a critical role in the apparent alterations of the uterus that are taking place during early pregnancy. Uterus undergoes functional and structural changes to become receptive to the invading blastocyst. Estrogen is also responsible for the control of the implantation.22 This hormone is also used during the transfer of embryos to assist the implantation. Different types of routes of administration and doses of estrogen are used along with progesterone. Receptive endometrium is an important factor for deciding the successful embryo implantation and receptivity to implantation can be achieved with the use of estrogen and progesterone given exogenously.23
Likewise, studies have also suggested that estrogen and progesterone take part in an important function of preparing endometrium to become receptive to embryo.24 They also stated that well-timed and appropriate initiation of endometrial receptivity is a key process for offering triumphant embryo implantation. A study by Young also concluded that estrogen is essential for endometrial receptivity to embryo implantation. This phase exists for a short period of time and can be accomplished only following adequate exposure.25 Klonos et.al., was also of the same opinion that both estrogen and progesterone help in achieving success in IVF process owing to their effects that lead to a succession of autocrine and paracrine signals. They are needed for processing of adhesion molecules for successful penetration and adherence of blastocytes to the endometrium.22
Furthermore, estrogen levels on the endometrium are also firmly regulated and in case of low level of estrogen, receptivity of the uterus persists and when levels go high, it is effects in role of endometrium. Thus, estrogen holds a fundamental and critical role in the establishment of receptivity of the endometrium and it can be said that success of IVF procedure depends on it.25
Endometriosis is sometimes the result of high levels of estrogen and is believed to enhance the growth of endometrial tissues in the body. Similarly, endometrial polyps are also sensitive to estrogen.
The study was conducted in collaboration with an IVF laboratory at a private medical center in Riyadh, Saudi Arabia. This was a case-control study, with case to control ratio of 5:1 i.e., for 50 patients with endometriosis, 10 patients without endometriosis served as control. The endometriosis was diagnosed by symptoms, and pelvic and transvaginal ultrasounds. The oocytes from all patients were fertilized by intracytoplasmic sperm injection and embryo transfers were carried out on day-5. The study parameters included measurement of serum estrogen and CA 125 marker levels, fertilization rate, pregnancy rate, implantation rate, and pelvic ultrasound observations. The measurement of CA 125 marker helped in differentiation of haemorrhagic cyst and the endometriosis masses. For detection of pregnancy, serum beta human chorionic gonadotropin (b-hCG) levels were determined using Abbott Architect. All data were recorded in Excel sheet for statistical analysis. The significance level was set at 0.05% for all statistical tests.
In patients without endometriosis, the average longitudinal measurement of the uterus was 7.5cm with a range of 5cm to 8.5cm and the average transverse measurement was 5.0cm with a range of 4.5cm-6.4cm (Figure 1).26 In the presence of endometriosis, this size increased depending on the size and site of the endometrioma. The Figure 2 shows dilation of the uterine cavity due to the presence of endometrioma. In this patient, the transverse measurement increased to 6.5cm (Figures 3a & 3b).
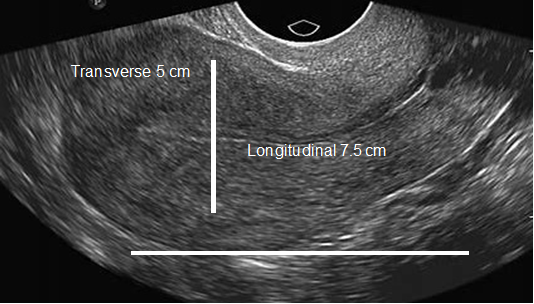
Figure 1 An ultrasound image of the uterus showing normal size. The horizontal line shows longitudinal measurement (7.5 cm), and the vertical line shows transverse measurement (5 cm).
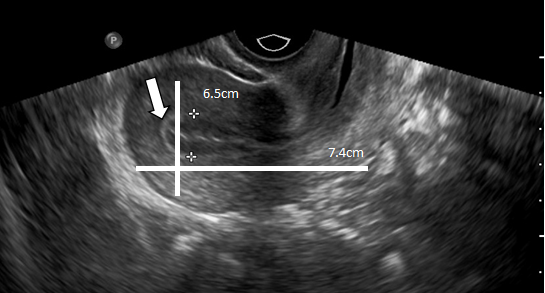
Figure 2 An ultrasound image of the uterus showing dilation of the uterine cavity due to the presence of an endometrioma.
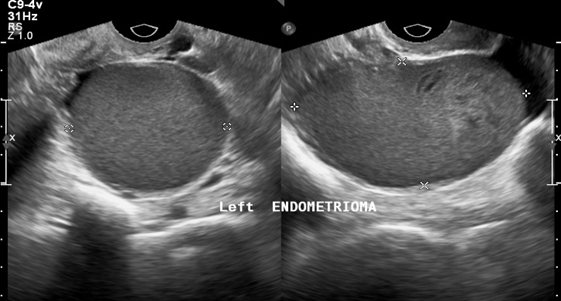
Figure 3a An ultrasound image of the uterus showing left endometrioma. The dots indicate size of masses present.
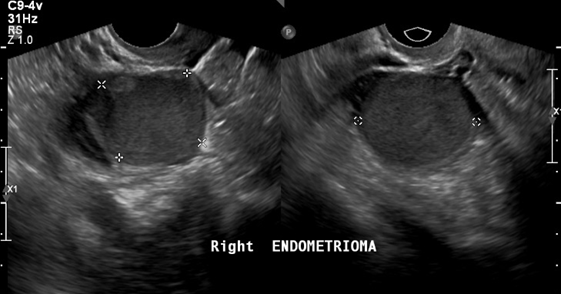
Figure 3b An ultrasound image of the uterus showing right endometrioma. The dots indicate size of masses present.
In all patients participating in this study, it was found that almost half 48.3% (n= 29) had high estrogen levels, 36.7% (n=22) had normal levels and 15% (n= 9) had low estrogen levels (Figure 4).
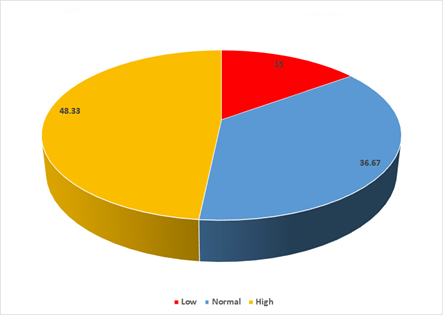
Figure 4 Estrogen levels in all patients participating in this study regardless of endometriosis. In this study, high estrogen levels were observed in 48.33 %, normal in 36.67 % and low in 15%).
In the endometriosis group, 28 patients had high estrogen levels and only 14 had normal levels, while the patients in the control group without endometriosis, only one patient had high estrogen level, and 8 had normal estrogen level (Figure 5).
In the endometriosis group, it was found that more than two thirds 68.37% (n=41) had a negative pregnancy test result, and 31.67% (n=19) had positive pregnancy results (Figure 6). Whereas in the control group, the biochemical pregnancy rate was 90%. These differences were significant.
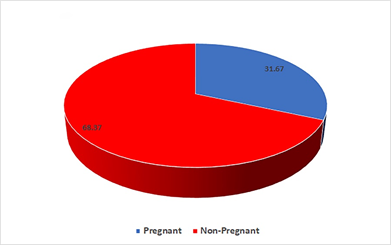
Figure 6 Showing pregnancy rate in patient suffering from endometriosis. A high percentage (68.33%) of patients in endometriosis group did not achieve pregnancy.
The blood level of CA125 marker was measured to differentiate between endometriosis and haemorrhagic cyst. Most of the patients with endometrioma had high levels of CA125 marker in the blood. The average CA125 value was 127.26±269U/ml, with a range of 37-1444U/ml whereas the normal value is 35 U/ml.
The results of this study indicate that patients suffering from endometriosis has significantly lower pregnancy rate after in vitro fertilization and embryo transfer stressing the need to seek medical treatment for endometriosis.27 In endometriosis cases, the lower pregnancy is mainly due to endocrine abnormalities, fibrosis, immune and inflammatory disorders. Since the pregnancy rate in IVF procedure is negatively correlated with endometriosis severity,28 therefore, a strategy to improve pregnancy rate in endometriosis cases is to defer the embryo transfer until the endometriosis is treated to improve the endometrial receptivity. Significant improvements in embryo vitrification had made it possible and many clinics have adopted the “freeze all” approach. It has now become an attractive approach to increase pregnancy rate in patients with endometriosis.29
The endometriosis causes pain and reduces reproductive potential, therefore, its treatment becomes essential. A common treatment is to use hormonal contraceptives or levonorgestrel-releasing intrauterine system. In severe cases, laparoscopic surgery is recommended to remove the endometriosis tissue. If the ovaries are involved, and have to be surgically incised or removed, oocyte cryopreservation to preserve fertility is recommended.30
During the diagnostic procedure some patients presented an ultrasonographic pattern that resembled a mass that was parallel to the ovaries. In such cases, CA125 marker levels were determined to rule out the presence of a haemorrhagic cyst. Normal values of CA125 marker are between 0 and 35U/ml. According to the current literature, endometriosis is a disease that triggers the production and release of this marker, so the patients with endometriosis exhibit high values of CA125 marker. The patients with CA125 marker values less than 35U/ml were considered to have a haemorrhagic cyst rather than endometriosis. The average value of CA125 marker in this study was 127U/ml which is higher than the value found in normal woman.
The estrogen levels in patients suffering from endometriosis were significantly higher than those in the control group. The endometriosis is estrogen-dependent disease. Its etiology includes interactions of genetic, immunological, hormonal and environmental factors. That is why no single theory can explain all aspects of endometriosis.31 The previous reports indicated that molecular and cellular features of endometriosis differ from those of endometrium.32,33 The aromatase and 17β-HSD type 1 mRNA levels are extremely low in normal human endometrium, however, in endometriosis, the enzymes producing estrogen are more active. This is due to a suppression of types 2 and 4 17β-HSD, and an increased expression of aromatase and type 1 17β-HSD in ectopic endometrium which results in higher levels of estrogen in endometriosis34 facilitating the implantation of endometrial fragments.
The results of this study indicated that the patients suffering from endometriosis exhibited higher estrogen and CA 125 marker values and had significantly lower implantation rate as compared to those in the control group. A larger study is warranted to confirm these findings.
None.
None.
Author declares there is no conflict of interest exists.

©2022 , et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.